Administration of red ginseng regulates microRNA expression in a mouse model of endometriosis
Article information
Abstract
Objective
Red ginseng (RG) exerts anti-inflammatory, anti-proliferative, and immunomodulatory effects on endometriosis through the regulation of microRNA (miRNA) expression. It may also ameliorate endometriosis by affecting the expression of multiple miRNAs simultaneously, rather than acting on a single miRNA at a given time. Since studies on the overall effects of RG on endometriosis via the regulation of miRNA expression are lacking, the current study aimed to explore the global effect of RG on miRNA expression in a mouse model of endometriosis.
Methods
To establish the mouse model, the uterine horn of donor mice was implanted into the lateral side of the recipients’ peritoneum, followed by vehicle or RG treatment for 8 weeks.
Results
To confirm the effects of RG on the established mouse model, the size of the implanted uterus was measured; it was found to be lower in mice from the RG group than in mice from the control group. miRNA expression profiles in the implanted uterus of the mouse model of endometriosis after vehicle or RG administration were analyzed using microarray technology. Thereafter, seven candidate miRNAs and 125 candidate genes (miRNA targets) were identified through a bioinformatics analysis.
Conclusion
The present findings suggest that RG regulates the expression of multiple miRNAs and mRNAs, thereby alleviating endometriosis in a mouse model of the disease.
Introduction
Endometriosis is a commonly occurring (affecting 6%–10% of women in the United States) chronic inflammatory disease, characterized by the formation of endometrial-like tissue outside the uterus, and associated with dysmenorrhea, dyspareunia, chronic pelvic pain, and infertility [1]. Sampson’s theory, suggesting retrograde menstruation via the fallopian tubes into the peritoneal cavity to implant on the surface of pelvic organs, is the most widely accepted hypothesis regarding the origin of ectopic endometrial tissue [2]. However, the exact cause of endometriosis is not fully understood. Since the incidence of retrograde menstruation is similar in women with and without endometriosis, numerous other mechanisms underlying endometriosis have also been proposed, such as coelomic metaplasia, altered immunity, and epigenetic modifications [3-5].
Recent research has focused on the contribution of epigenetic mechanisms, including DNA methylation, histone acetylation, and changes in co-regulators, such as microRNAs (miRNAs), to the development of endometriosis [6-8]. In particular, miRNAs, which are involved in mRNA silencing and post-transcriptional regulation of gene expression, have been reported to be aberrantly expressed in patients with endometriosis [9-11]. A recent prospective case-control study using miRNA microarray and quantitative polymerase chain reaction analyses of serum samples from women with or without endometriosis reported abnormally regulated miRNA expression in women with endometriosis, thus suggesting the possibility of a novel non-invasive diagnostic test for the early detection of endometriosis [10-12].
Panax ginseng, which has been used in traditional medicine for centuries [13], is being actively studied for its medical effectiveness in modern clinical research. Among its various types, red ginseng (RG; P. ginseng Meyer) is prepared by steaming and drying fresh P. ginseng to prevent its rapid decay. During this processing, the properties of ginsenosides are chemically transformed, resulting in a pharmacological efficacy different from that of the original P. ginseng [14-16]. Recent studies have proven that RG exerts various medical effects, including anti-inflammatory, antioxidant, and immunomodulatory effects, along with epigenetic regulation, in various diseases [16-18]. Moreover, RG exerts anti-tumor effects in endometriosis by inhibiting angiogenesis and cell migration in vitro and in vivo [19,20].
Because endometriosis occurs spontaneously only in humans and some non-human primates, an increasing number of studies have attempted to establish models for endometriosis using small laboratory animals, especially rodents, by transplanting pieces of endometrial tissue to ectopic sites [21]. In particular, animal models are often used to assess endometriosis to confirm the efficacy of drugs in vivo or to characterize epigenetic changes resulting from various interventions [22-24]. These animal models of induced endometriosis have proven to be useful in the evaluation of the mechanisms underlying the pathogenesis of this complex disease [21].
We previously reported that ginsenoside Rg3 of RG decreases the fibrotic and invasive nature of endometriosis by modulating miRNA-27b expression in a mouse model of endometriosis [19]. Although we previously investigated the regulatory effect of RG on miRNA-27b, we now hypothesize that RG may ameliorate endometriosis by simultaneously regulating the expression of multiple miRNAs, rather than that of a single miRNA at a time. Hence, in this study, we analyzed the expression levels of various miRNAs isolated from a mouse model of endometriosis after RG administration and performed bioinformatics analyses to investigate the global effect of RG on endometriosis in vivo.
Methods
1. Ethics statement and animal care
This study was approved by the Institutional Animal Care and Use Committee and the Institutional Committee on Animal Care of Gangnam Severance Hospital, Yonsei University Health System (approval No. 3-2015-0007). All animal experiments were performed in accordance with the animal research: reporting of in vivo experiments (ARRIVE) guidelines [25]. The mice used herein were purchased from Orient-Bio Laboratory Animal Co., Ltd. (Seongnam, Korea). They had ad libitum access to food and water, and were housed in a polyacrylic cage under controlled conditions at 24°C±1°C, with 50%±5% humidity and a 12-hour day/night cycle.
During the surgical establishment of the endometriosis model, the mice were placed in a Harvard-type isoflurane chamber, and 3.5%–4% isoflurane (Hana Pharmaceutical Co., Ltd., Seoul, South Korea) was administered in conjunction with air for anesthesia induction. Thereafter, 1.5%–3% isoflurane was administered to maintain anesthesia in mice. Their breathing was carefully observed, and in cases of respiratory depression, oxygen levels were increased in the chamber because isoflurane anesthesia is rapidly recoverable [26]. To prevent infection, 10 mg/kg cefazolin sodium (Korea United Pharm, Seoul, Korea) was intravenously administered before the procedure. After all animal experiments, the mice were euthanized. To decrease pain and animal distress, the experimental animals were treated with 6%–7% isoflurane until 2 minutes after breathing cessation, and euthanasia was performed through cervical dislocation.
2. Mouse model of endometriosis and RG administration
A mouse model of endometriosis was established using 6-week-old C57b/6 female mice (n=9) having a similar weight (28±4 g) as described previously [11]. In brief, each donor mouse (n=3) was sacrificed following an anesthetic overdose and its uterus was harvested. The “Y”-shaped uterus was cut in half, and each uterine horn was dissected to expose the endometrium. The recipient mouse was anesthetized, and each uterine horn was transplanted into the corresponding lateral side of the recipient’s peritoneum using Vicryl 3-0 sutures. Before transplantation, a cytological evaluation of vaginal smears from virgin female mice was used to determine the current estrous stage. The peritoneum and skin were closed with Vicryl 3-0 sutures and staplers, and the postoperative condition of the mice was examined. RG extract, provided by Korea Ginseng Corporation, was diluted in PBS, as described previously [19]. The experimental animals were randomly divided into two groups—vehicle and RG—each comprising three mice. The RG group was administered 0.2 mg/g RG extract by oral gavage once a day; similarly, the control vehicle group was administered an equivalent amount of water. RG administration was initiated 1 day after transplantation and was continued for 8 weeks. Thereafter, all mice were sacrificed for endometrial tissue retrieval. The area of endometrial implants was measured based on the length and width of each lesion and calculated using ImageJ (version 1.41o, Java 1.6.0_10, Wayne Rasband; US National Institutes of Health, Bethesda, MD, USA).
3. Histological examination
The endometrial implants were collected, fixed in 10% formalin-acetic acid, and embedded in paraffin for histopathological examination. Sections of paraffin-embedded tissue were stained with Masson trichrome. To quantify the severity of fibrosis in stained tissue sections, staining intensity was measured using ImageJ, and the severity of fibrosis was evaluated according to the Ashcroft method [27].
4. Isolation of miRNAs from endometrial implants
Total RNA was isolated using TRIzol (Invitrogen; Thermo Fisher Scientific, Waltham, MA, USA) and purified using the RNeasy mini kit (Qiagen, Hilden, Germany), according to the manufacturer’s instructions. RNA quality and quantity were estimated using an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA) and ND-1000 Spectrophotometer (NanoDrop, Wilmington, DE, USA), respectively.
5. Affymetrix miRNA arrays
The Affymetrix GeneChip miRNA 4.0 array process was carried out according to the manufacturer’s protocol (Affymetrix, Santa Clara, CA, USA). Approximately 1,000 ng of RNA was labeled with the FlashTag Biotin RNA Labeling Kit (Genisphere, Hatfield, PA, USA). The labeled RNA was then quantified, fractionated, and hybridized to the miRNA microarray chip according to the standard procedures recommended by the manufacturer. The labeled RNA was heated to 99°C for 5 minutes and then to 45°C for another 5 minutes. RNA-array hybridization was performed in an Affymetrix Fluidics Station 450 with agitation at 60 rotations per minute for 16 hours at 48°C. The chips were washed and stained using a GeneChip Fluidics Station 450 (Affymetrix) and then scanned with an Affymetrix GCS 3000 scanner. Signals were quantified using the Affymetrix GeneChip Command Console (AGCC) software.
6. Bioinformatics prediction
The predicted target genes of candidate miRNAs were determined using three bioinformatics prediction tools: TargetScan v6.2 (http://www.targetscan.org/mamm_31/), miRmap (http://mirmap.ezlab.org/), and miRDB (http://www.mirdb.org/miRDB/). The selection criteria were as follows: correlation >0.99 or <–0.99 and a p-value <0.05. The genes that overlapped in all three databases were selected for further functional analyses. The miRNAs and predicted mRNA target genes were then subjected to Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses using David v6.7 (http://david.abcc.ncifcrf.gov/) online. The predicted target gene list of differently expressed miRNAs obtained as described above was entered into the Cytoscape software (version 3.4.0), and the connections between miRNAs and their predicted gene targets were visualized.
7. Raw data preparation and statistical analysis
Raw data were extracted automatically via the Affymetrix data extraction protocol using the software provided by the AGCC. The CEL file import, miRNA level RMA+DABG-all analysis, and results export were performed using Affymetrix Expression Console software. Array data were filtered using probe-annotated species. Comparative analysis between the test and control samples was conducted using the independent t-test and fold changes, in which the null hypothesis was that no difference existed between the groups. The false discovery rate was controlled by adjusting the p-value using the Benjamini-Hochberg algorithm. All statistical tests and visualization of differentially expressed genes were conducted using the statistical language R v3.1.2. (www.r-project.org).
Results
1. RG administration attenuated the symptoms of endometriosis
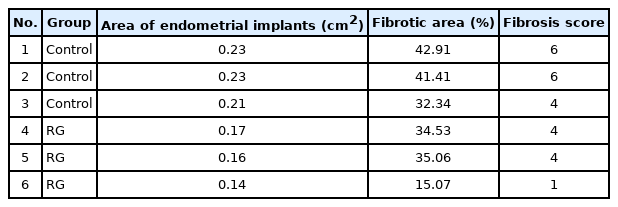
After 8 weeks of RG administration, the mice were sacrificed and their endometrial lesions were analyzed. All transplanted endometrial lesions were found in the peritoneum of the sacrificed mice. The mean areas of lesions in the vehicle (n=3/3) and RG (n=3/3) groups were 0.22±0.03 cm2 and 0.14±0.03 cm2, respectively. The mean area of endometrial implants was lower in the RG group than in the vehicle group (p=0.043) (Figure 1, Table 1). A histological analysis was carried out to evaluate the effects of RG administration on the endometrial lesions, and a reduction of the mean area and fibrosis scores were observed in the implants of RG group (p<0.001) (Figure 1, Table 1).
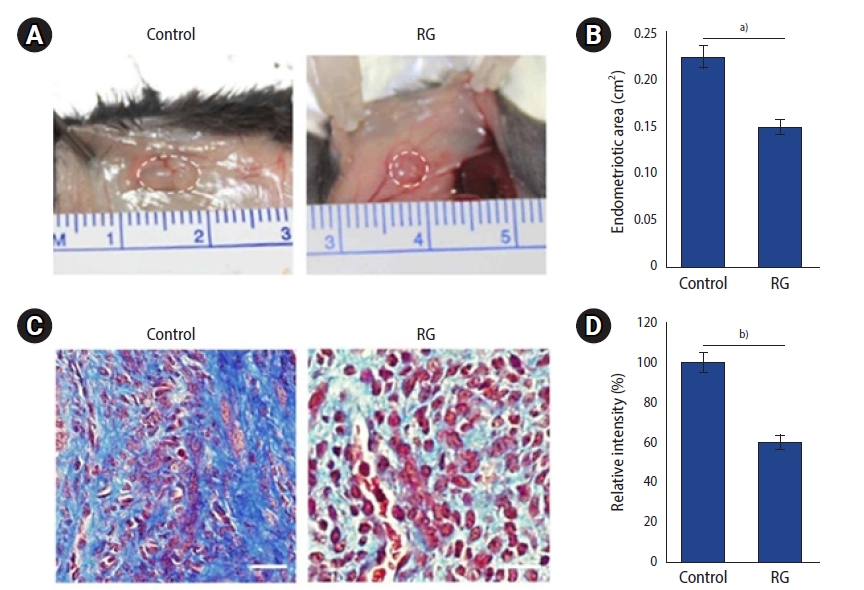
Results from the mouse model of endometriosis. (A) The circles indicate endometrial implants in the mouse model from the control group (vehicle) and the red ginseng (RG) group (red ginseng extract 0.2 mg/g) after 8 weeks of treatment. (B) The endometrial implants of mice from the RG group were significantly smaller than those of mice from the control group. (C) Masson trichrome staining of endometrial implants from both control and RG groups (scale bar=20 μm). (D) The staining intensity of samples from the RG group was significantly lower than that of samples from the control group. Control vs. RG, a)p<0.05 and b)p<0.01, n=3 per group.
2. RG administration altered miRNA expression in the mouse model of endometriosis
miRNA array data were analyzed to detect the expression profiles of miRNAs in endometrial implants isolated from the mice in the two groups to examine whether RG administration could alter the miRNA expression profile. Seven miRNAs were observed to be differentially expressed (fold change ≥1.5) in the RG group compared to the control group (Figure 2). Expression data were normalized using the median normalization method, after which the differentially expressed miRNAs between two samples were filtered based on fold change. The miRNA array results, obtained after normalization, and the calculated fold change values are listed in Table 2. The significant difference in the expression profiles between the two groups suggested that RG administration altered the expression of miRNAs in the mouse model of endometriosis.

Heat map of microRNA (miRNA) profiling after the different treatments. Hierarchical clustering shows seven significantly regulated miRNAs in the red ginseng (RG) and control groups. A total of six miRNAs were downregulated and one was upregulated in the control group compared to the RG group. The color code in the heat maps is linear, with yellow and blue indicating the lowest and highest expression, respectively.
3. GO and KEGG analyses of miRNAs
To determine the potential effect of differentially expressed miRNAs following RG administration in the mouse model of endometriosis, the probable target genes of these miRNAs were predicted through TargetScan v6.2 and DIANA miRPath v.3 analyses. The target genes were significantly overrepresented in GO processes associated with molecular function, such as protein binding, ion binding, organic cyclic compound binding, heterocyclic compound binding, catalytic activity, cation binding, metal ion binding, nucleic acid binding, and enzyme binding (Figure 3). In addition, representative pathways included the metabolic, Ras, Rap1, mitogen-activated protein kinase (MAPK), Axon, PI3K-Akt, and chemokine signaling pathways (Figure 3).
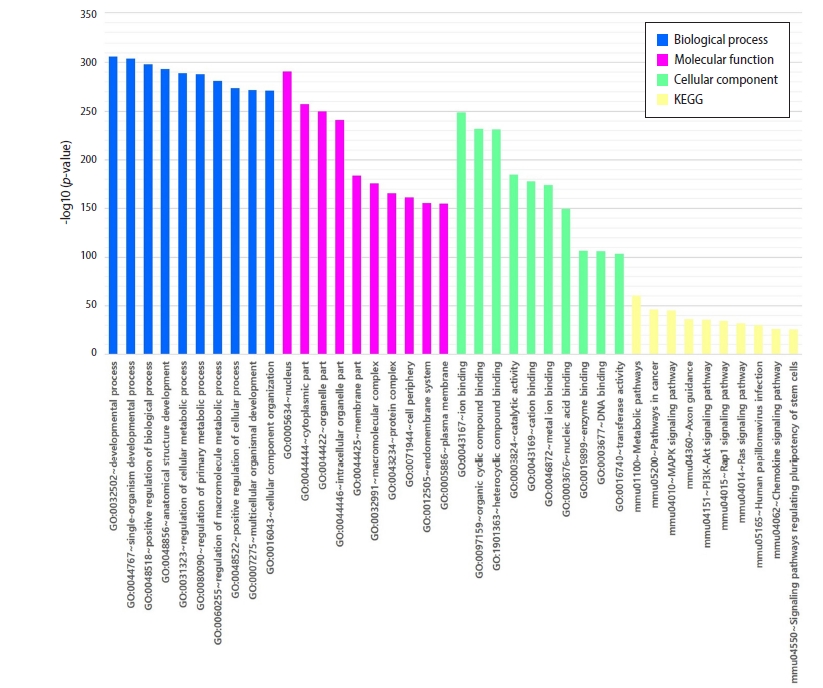
Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis of candidate microRNA (miRNA) target genes. The top 10 significant GO and KEGG terms of the altered miRNA target genes are shown. The shared GO terms of the “Up/Down and Down/Up” microRNA target genes are associated with protein binding, ion binding, organic cyclic compound binding, heterocyclic compound binding, catalytic activity, cation binding, metal ion binding, nucleic acid binding, enzyme binding (molecular function), membrane-bound organelle, intracellular organelle, and membrane (cellular component).
4. Integrated analysis of miRNAs and mRNAs
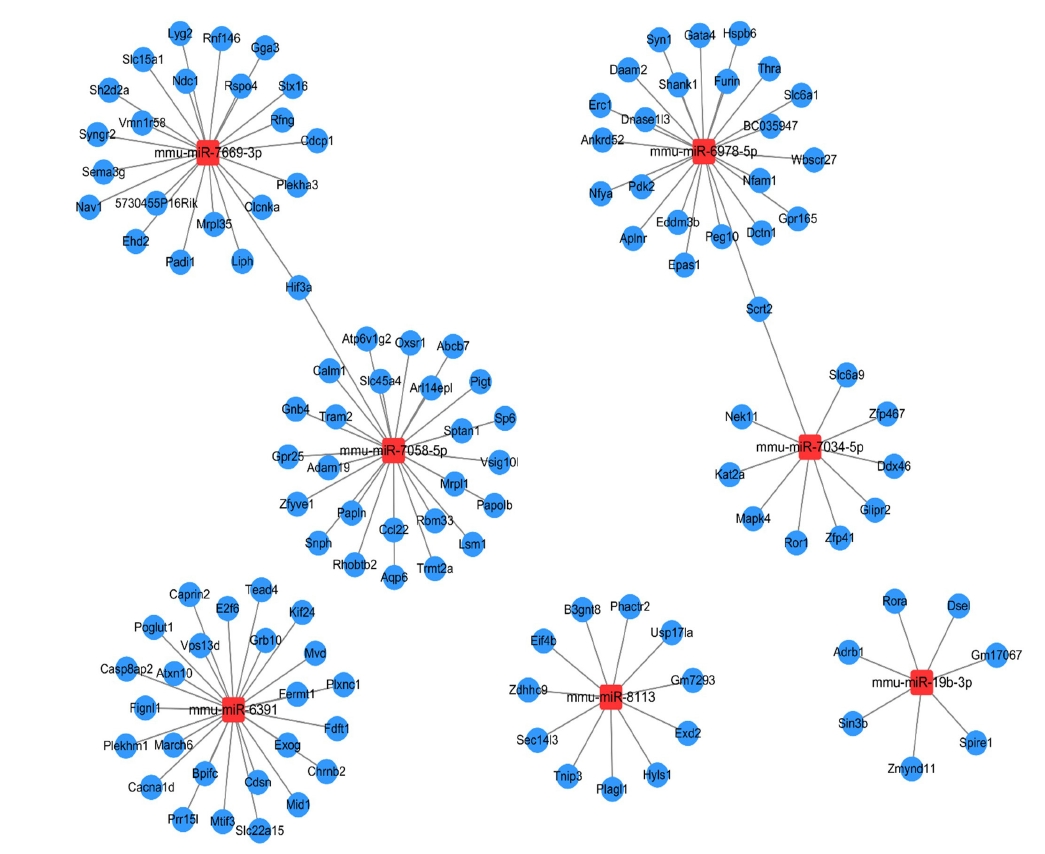
Because each miRNA has multiple potential mRNA targets, a single mRNA can be regulated by multiple miRNAs. The 7 miRNAs observed to be differentially expressed were further analyzed, and their 125 predicted target genes were screened (miRNA-target gene cutoff: TargetScan context score <–0.6; visualization tool: Cytoscape 3.7.1). The results demonstrated that two genes, Hif3a and Scrt2, were co-regulated by at least two miRNAs (Figure 4).
Discussion
This study investigated the molecular network of miRNA and mRNA expression that modulates critical processes in a mouse model of endometriosis following RG administration. To clarify the influence of miRNAs and mRNAs on the mouse model, a group of mice was treated with RG and the differences in miRNA expression between these mice and control mice were evaluated by microarray analysis. Along with bioinformatics-based predictions, RG administration was found to alter the expression profile of multiple miRNAs in the endometriotic lesions. Among them, seven miRNAs and 125 candidate genes were involved in the main microRNA-mRNA regulatory mechanism.
This study successfully established a mouse model of endometriosis and accompanying fibrosis, as confirmed by Masson trichrome staining. RG administration significantly reduced the size of the endometrial implants compared to that in the control group. As is commonly observed, alterations in the morphology and size of various organs are accompanied by changes in gene expression. One of our previous studies suggested that ginseng induced apoptosis in endometrial cells derived from patients with endometriosis via the regulation of miR-21-5p expression [28]. In another study, we reported that RG administration changed the fibrotic properties of human endometrial stromal cells by regulating miR-27b-3p levels in vivo [19].
The results from the miRNA array analysis in this study revealed seven miRNAs to be differentially expressed in the RG group compared to the control group. Through GO and KEGG analysis, we identified the molecular miRNA-mRNA expression network that regulated protein binding, metabolic pathways (including Ras, Rap1, MAPK, Axon, and PI3K-Akt), and chemokine signaling pathways in the mouse model of endometriosis following RG administration. In fact, most of these pathways have already been reported to play an important role in endometriosis. Increased activation of the Ras/Raf/MAPK pathway is known to be associated with the enhanced proliferation and survival of eutopic endometrial cells (from patients with endometriosis) in vitro [29]. Many reports have already suggested that the PI3K/Akt/mTOR pathway is associated with the development of endometriosis. In vivo studies have shown that the mTOR/AKT inhibitor temsirolimus prevents deep infiltrating endometriosis, which is the most severe form of endometriosis [30,31]. Moreover, ginsenoside Rg3 has been reported to inhibit the angiogenesis associated with endometriosis through the vascular endothelial growth factor receptor-2-mediated PI3K/Akt/mTOR signaling pathway in a rat model [20]. RG administration is thought to have reduced the growth of ectopic endometriotic tissue by modulating the expression of miRNAs targeting the PI3K/Akt/mTOR pathway in this animal-based study.
As mentioned above, endometriosis is associated with altered immunity, suggesting that impaired immune function may contribute to the development of this disease. Instead of acting as scavengers to eliminate ectopic endometrial cells, activated macrophages and circulating monocytes in women with endometriosis appear to promote the disease by secreting growth factors and cytokines (interleukin [IL]-1, IL-6, and IL-8, tumor necrosis factor, regulated on activation, normal T cell expressed and secreted, and vascular endothelial growth factor) that stimulate the proliferation of ectopic endometrial tissue and inhibit their scavenging functions [32-34]. Based on the results of this study, RG may affect proliferation and mitosis of ectopic endometrial cells of mice, and seems to act on the scavenging mechanism by which endometriosis is eliminated from the body. There is sufficient evidence for alterations in both cellular and humoral immunity in women with endometriosis, thus implying that endometriosis is the result of dysregulated chemotaxis [35].
Ginseng has been extensively reported to maintain the homeostasis of the immune system and enhance resistance to inflammation and microbial attacks through the regulation of the immune system [36]. Rg3, one of the main components of RG, has been reported to induce immunogenic cell death in tumor cell lines (B16F10 melanoma cells) by suppressing pro-angiogenic (tumor necrosis factor-α) and immunosuppressive cytokine (transforming growth factor-β) secretion, as well as interferon-gamma production from Rg3-treated tumor cells [37]. In another study, RG extract was shown to inhibit the apoptosis of cells from a pancreatic β-cell line (MIN6N8), possibly via the reduction of nitric oxide and reactive oxygen species production, inhibition of p53/p21 expression, and inhibition of cleavage of caspases and poly(ADP-ribose) polymerase; this suggests the RG-mediated inhibition of cytokine-induced apoptosis in pancreatic β-cells [38]. Collectively, RG may regulate the expression of several miRNAs involved in the Ras/Raf/MAPK and PI3K/Akt/mTOR pathways and immune system regulation in a mouse model of endometriosis.
In this study, an integrated analysis of miRNAs and mRNAs demonstrated that two genes, HIF3a and SCRT2, were co-regulated by at least two miRNAs (Figure 4). Neither the genes nor the pathways containing the genes have been reported to be associated with endometriosis in the previous literature. The relationships between the two genes and endometriosis are not yet understood; however, such a relationship seems reasonable, owing to the tissue-specific nature of miRNA expression [39]. For example, the HIF3a gene is known to exhibit high miRNA-related expression levels in the bone, vessels, and prostate [40-42]; however, thus far, there are very few reports of its expression in the endometrium.
This study has several strengths. It describes the successful establishment of a mouse model of endometriosis and reports the global effects of RG on the miRNA-mRNA expression network in the model. Furthermore, various miRNA target genes were evaluated through bioinformatics analyses. These results suggest that RG potentially influences endometriosis via various target genes. However, this study has some limitations. First, considering the limitations of animal-based studies, it is difficult to interpret these findings as indicative of the efficacy of RG among endometriosis patients. Second, because the experiment included only a few animals, to minimize the sacrifice of experimental animals, there may be some differences among the miRNAs and mRNAs that show altered expression following RG treatment when compared to experiments performed using a large number of animals. Third, there are limitations in applying the results of this study to humans directly, because the mouse model of endometriosis was established in different way from endometriosis development in the human body. Moreover, miRNAs of mice (Mmu-miR) were analyzed as outcomes in this study. Nevertheless, it is known that genetic variation is similar between endometriosis in mouse models and endometriosis in humans. For example, when a gonadotropin-releasing hormone agonist was administered in an endometriosis animal model, the expression of matrix metalloproteinases and matrix metalloproteinase inhibitors, which are known to affect the pathogenesis of human endometriosis, was also changed [43]. Due to the similarity between endometriosis in murine models and humans, endometriosis murine models have been used in many studies as a test-bed for potential therapeutics including melatonin [44], doxycycline [45], etanercept (an anti-tumor necrosis factor-α antibody) [46], and gene therapy delivered via polymeric micelles [47].
In conclusion, RG administration may be considered to have significant effects on reducing the size of endometrial implants besides regulating miRNA expression levels in a mouse model of endometriosis. Functional analysis indicated that altered miRNA expression is involved in multiple pathways, including the Ras/Raf/MAPK and PI3K/Akt/mTOR pathways, and immune system regulation; targeting these pathways may eventually alleviate the symptoms of endometriosis, thereby inhibiting progression of the disease.
Notes
Conflict of interest
This study was financially supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI18C2047). The authors declare that they have no conflict of interest. The funders played no role in the study design, the collection, analysis, and interpretation of data, and the writing of the manuscript, which were completely the responsibilities of the authors.
Author contributions
Conceptualization: SC, JHL. Formal analysis: SC, JHL. Investigation: JHP, BHW, WI. Writing–original draft: JHL. Writing–review & editing: all authors.