Subcutaneous progesterone versus vaginal progesterone for luteal phase support in in vitro fertilization: A retrospective analysis from daily clinical practice
Article information
Abstract
Objective
Progesterone application for luteal phase support is a well-established concept in in vitro fertilization (IVF) treatment. Water-soluble subcutaneous progesterone injections have shown pregnancy rates equivalent to those observed in patients receiving vaginal administration in randomized controlled trials. Our study aimed to investigate whether the results from those pivotal trials could be reproduced in daily clinical practice in an unselected patient population.
Methods
In this retrospective cohort study in non-standardized daily clinical practice, we compared 273 IVF cycles from 195 women undergoing IVF at our center for luteal phase support with vaginal administration of 200 mg of micronized progesterone three times daily or subcutaneous injection of 25 mg of progesterone per day.
Results
Various patient characteristics including age, weight, height, number of oocytes, and body mass index were similar between both groups. We observed no significant differences in the clinical pregnancy rate (CPR) per treatment cycle between the subcutaneous (39.9%) and vaginal group (36.5%) (p=0.630). Covariate analysis showed significant correlations of the number of transferred embryos and the total dosage of stimulation medication with the CPR. However, after adjustment of the CPR for these covariates using a regression model, no significant difference was observed between the two groups (odds ratio, 0.956; 95% confidence interval, 0.512–1.786; p=0.888).
Conclusion
In agreement with randomized controlled trials in study populations with strict selection criteria, our study determined that subcutaneous progesterone was equally effective as vaginally applied progesterone in daily clinical practice in an unselected patient population.
Introduction
Luteal phase defects (LPDs) are a common issue in stimulated in vitro fertilization (IVF) cycles. The main causes of LPDs include supra-physiological concentrations of steroids, inhibition of luteinizing hormone (LH) release, premature luteolysis, and loss of progesterone synthesis [1]. Therefore, it is essential to supply women undergoing IVF with exogenous progesterone or human chorionic gonadotropin (hCG) [2]. As hCG injections are equally effective, but are associated with a higher risk of ovarian hyperstimulation syndrome, progesterone has become the treatment of choice for luteal phase support in assisted reproduction [3,4].
Progesterone can be applied orally, vaginally, intramuscularly, or subcutaneously, and each route has certain advantages and disadvantages. Subcutaneous progesterone injection was introduced as a water-soluble compound at a daily dose of 25 mg in 2014 [5]. Clinical trials revealed that a daily dose of 25 mg of subcutaneous progesterone is sufficient to reach endometrial receptivity, even in the absence of endogenous progesterone [6]. A prospective study on patients’ opinions demonstrated a higher level of satisfaction in patients undergoing subcutaneous progesterone injections than among those undergoing administration through the vaginal route [7]. Although vaginal administration is widely used in the majority of IVF centers globally, a reasonable number of patients prefer injections [8-10]. Subcutaneous progesterone has been studied and approved based on two large, randomized, controlled phase III trials in Europe and the USA during 2013, establishing that patients undergoing IVF treated with subcutaneous progesterone injections exhibited similar pregnancy rates to those of patients using a vaginal gel or tablets [11,12].
At the time of the study, our center administered either vaginal micronized progesterone at 600 mg per day or, at patients’ discretion, daily subcutaneous progesterone. Our study was intended to investigate whether the results from earlier pivotal trials could be reproduced in daily clinical practice in an unselected patient population to establish whether subcutaneous progesterone is as effective as vaginal progesterone and results in the same pregnancy rates.
Methods
1. Study design and participants
This retrospective study was based on a cohort undergoing all stimulation cycles for IVF or intracytoplasmic sperm injection (ICSI) treatment between July 23, 2015, and February 11, 2017, with at least one embryo transfer at a university hospital in Marburg, Germany. Patients were included irrespective of previous treatment cycles, underlying diagnoses, current stimulation regimes, or dosing. Frozen embryo transfer cycles and patients with an endometrial thickness <6 mm were not included.
Appropriate, individualized stimulation procedures were selected based on patient-specific characteristics, including anti-Müllerian hormone level, weight, and antral follicle count at the beginning of the stimulation, as well as preliminary results from previous stimulation cycles. Patients received for luteal phase support either vaginal administration of 200 mg of micronized progesterone three times daily using Progestan (Dr. KADE/BESINS Pharma, Berlin, Germany), Utrogestan (Kohlpharma, Merzig, Germany) and Famenita (Exeltis Germany, Ismaning, Germany) or 25 mg of a water-soluble and subcutaneous injectable complex of progesterone (Prolutex; Marckyrl Pharma, Papenburg, Germany) once daily. The clinical pregnancy rate (CPR) was calculated based on successful pregnancies, which were determined by sonographically verified evidence of a gestational sac, per transfer.
The data used in this study were collected as part of clinical treatment processes using the internal management and documentation program Meditex IVF (Critex, Regensburg, Germany). Patient baseline data, sterility-related factors, and any known previous treatments were recorded during clinical routines and analyzed anonymously according to local and European ethics and data protection regulations. As part of a routine clinical follow-up, pregnancies and births were documented according to Germany’s IVF registry and quality management obligations.
2. Stimulation protocol
In the short protocol, the ovarian stimulation started on the second or third day of the cycle using stimulation pens and injection accessories. The stimulation drugs used in this study were recombinant follicle-stimulating hormone (rFSH; Gonal F, Merck Serono, Darmstadt, Germany or Ovaleap, Teva, Ulm, Germany or Puregon, MSD Sharp & Dohme, Haar, Germany) and/or human menopausal gonadotropin (Menogon; Ferring, Kiel, Germany). Some of the patients also received rFSH and recombinant LH (Pergoveris, Merck Serono).
In the long protocol, a GnRH analogue was administered in the month preceding the stimulation from the middle of the luteal phase. The active ingredient nafarelin (Synarela; Pfizer, New York, NY, USA) was used in a nasal application form at 0.4 mg/day. The subgroup of female patients who underwent a natural cycle received either no stimulation or oral stimulation using clomiphene citrate, a selective estrogen receptor modulator (Clomifen, Ferring).
3. Statistical analysis
The two-tailed t-test was used to analyze and compare the two groups’ baseline characteristics. A mixed logistic regression model was used to compare the per-cycle pregnancy rates between the subcutaneous and vaginal groups while accounting for intra-patient correlations between multiple cycles in the same patients. The regression model was expanded to include each of the potential predictors: age, body mass index (BMI), transfer day, number of embryos transferred, medications used (stimulation), total stimulation medication dosage, and the stimulation protocol. We used a model including all of the predictors listed to account for confounding in comparisons of pregnancy between the subcutaneous and vaginal groups. The two-tailed t-test was conducted using IBM SPSS ver. 27 (IBM Corp., Armonk, NY, USA). The R programming environment was applied for the mixed logistic regression model.
Results
During the study period, 195 women met the inclusion criteria of the study. The women underwent 273 IVF cycles. In 197 cycles, the women received vaginal micronized progesterone in soft capsules (200 mg of Progestan, Utrogestan, Famenita) three times daily, and in 76 cycles, they received daily subcutaneous progesterone through injections of an aqueous solution (Prolutex, 25 mg). The treatments started on the day of oocyte aspiration and lasted until 14 days after transfer or, if there was a positive pregnancy test, up to the 12th week of gestation.
Patients’ demographic and baseline characteristics are presented in Table 1. No significant differences were observed between the treatment groups in age, weight, height, number of oocytes, number of inseminated oocytes, or the rate of blastocyst transfer. The mean BMI values were normal (<25 kg/m2) and comparable between the two groups (24.4±4.48 kg/m2 in the vaginal group and 24.8±4.94 kg/m2 in the subcutaneous group) (Table 1).
In the subcutaneous progesterone group, 31.6% of embryos were transferred at the cleavage stage and 68.4% at the blastocyst stage, while these proportions in the vaginal progesterone group were 55.8% at the cleavage stage and 44.2% at the blastocyst stage. The stimulation characteristics (medication and protocol) varied between the treatment groups. However, the total doses of FSH stimulation medication used in the two groups were similar (2,217.4±989.3 IU/mL in the vaginal group vs. 2,297±692.9 IU/mL in the subcutaneous group) (Table 1).
There was no significant difference in the CPR per treatment cycle between the subcutaneous (39.9%) and vaginal groups (36.5%, p=0.630) (Table 2). Moreover, the rates of live births per embryo transfer (28.9% in the vaginal group vs. 30.2% in the subcutaneous group, p=0.887), implantation (25.8% in the vaginal group vs. 32.2% in the subcutaneous group, p=0.261) and early spontaneous abortion (35% in the vaginal group vs. 39.5% in the subcutaneous group, p=0.386) were also similar between the two groups (Table 2).
Investigating the influence of clinical covariates on pregnancy outcomes showed that the number of transferred embryos, embryo transfer day, and total dosage of stimulation medication correlated significantly with the CPR (Table 3). However, when the CPR was adjusted for these covariates using the regression model, no statistically significant difference was found between the subcutaneous and vaginal groups (odds ratio, 0.956; 95% confidence interval, 0.512–1.786; p=0.888) (Table 4).
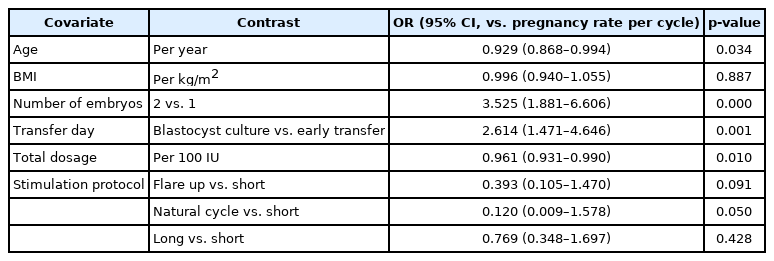
Odds ratio and 95% CI for contrasts according to each of the covariates included in the model of the pregnancy rate per cycle
Discussion
The results of our study showed that subcutaneous injections of progesterone were as effective as vaginal application in terms of the CPR in daily clinical practice in an unselected patient population.
This study was intended to evaluate differences in the CPR between groups of unselected patients receiving either subcutaneous injections or transvaginal insertion of progesterone for luteal phase support in daily clinical practice. The groups were comparable in terms of their baseline characteristics. As expected from the pivotal studies, the CPR did not differ significantly in simple comparisons (Table 2). After adjustment for confounding factors, the slightly higher rate in the subcutaneous group remained nonsignificant (Table 4).
In 2014, Lockwood et al. [11] reported the first phase III randomized study in Europe to determine the safety, tolerability, and efficacy of the subcutaneous progesterone formulation compared with standard vaginal progesterone gel for luteal phase support in women undergoing IVF/ICSI cycles. In that study, the ongoing pregnancy rate per retrieval was similar between groups receiving 25 mg of subcutaneous Prolutex (27.4%) and 90 mg of vaginal Crinone 8% gel (30.5%). In the same year, Baker et al. [12] recruited 800 women in the United States undergoing IVF for the second phase III randomized multicenter trial, comparing 25 mg of subcutaneous progesterone (Prolutex) to 100 mg of vaginal micronized progesterone (Endometrin) twice daily. Despite differences in the vaginal preparations between the two studies, there were no significant differences in clinical outcomes, including the pregnancy rate per retrieval, between subcutaneous (41.6%) and vaginal (44.4%) groups [12], indicating that subcutaneous application is not inferior to vaginal progesterone for luteal phase support.
These studies were the basis of the approval of subcutaneous progesterone for treatment. Randomized clinical trials are needed to evaluate medical outcomes, but those studies need to be done with highly specific patient populations. To our knowledge, no publications to date have confirmed the reproductive results of a randomized controlled trial in daily clinical practice. Of note, Baker et al. [12] used 200 mg of Endometrin daily in the US trial, whereas the recommended dosage of vaginal micronized progesterone is 600 mg daily. This may be due to the fact that the method and dosage of vaginal application appear to be irrelevant in luteal phase supplementation [13]; however, as micronized vaginal progesterone is widely used, it is of interest to compare the recommended 600 mg daily dosage of vaginal micronized progesterone to subcutaneous application. Therefore, our study is the first of its kind to demonstrate no significant difference in the CPR between unselected patient cohorts receiving 25 mg subcutaneous injections (39.9%) or 600 mg of micronized vaginal progesterone (36.5%) in daily clinical practice. BMI, as a critical factor in assisted reproductive technology (a BMI ≥25 kg/m2 leads to statistically significantly lower live birth rates) [14], was normal in our cohort (24.4 kg/m2 in the vaginal group and 24.8 kg/m2 in the subcutaneous group) (Table 1). The BMI values were also normal and similar between groups in both the European and U.S. trials [11,12], so there is limited knowledge of the possible impacts of being overweight on pregnancy outcomes with subcutaneous progesterone. Further studies could be designed with a broader range of BMI to investigate possible differences between progesterone types used for luteal phase support in IVF for overweight patients [15,16].
This study faces several limitations. First of all, the retrospective design does not allow any causal conclusions, and the control of bias was limited. After adjustment for confounding factors, patients on subcutaneous progesterone showed a nonsignificantly different CPR (Table 4). The nonsignificant difference in CPR might well point in a certain direction, and further studies on subgroups such as older women or obese patients might show relevant differences in pregnancy outcomes. This study was obviously not randomized. Patients were offered both options and given neutral explanations of the advantages and disadvantages of each treatment, but a subtle selection bias cannot be eliminated. However, the comparison of baseline data showed no major differences between the groups. Subcutaneously treated patients were slightly younger (35.2±3.63 vs. 36.2±4.21 years), received a few more oocytes (8.58±4.14 vs. 7.71±4.69), and had slightly more blastocyst transfers (68.4% vs. 55.8%). These might well explain the somewhat higher CPR in the unadjusted comparison (Table 2). After adjustment for these critical factors, a nonsignificant difference in the CPR was shown. In addition, the live birth rate per embryo transfer (28.9% vs. 30.2%) and the implantation rate (25.8% vs. 32.2%) did not differ significantly between the two groups. This should encourage further research, as there might be a particular population that could benefit from one or the other treatment.
In conclusion, our study confirms the results of pivotal RCTs in clinical practice through a comparison of subcutaneous progesterone to vaginal micronized progesterone (200 mg of Progestan, Utrogestan, Famenita, three times daily). Recent reviews of multiple clinical trials have shown that using different progesterone formulations for luteal support did not affect the pregnancy rates [17]. Therefore, women undergoing IVF have multiple choices for an appropriate progesterone administration route, and subcutaneous application appears to be an effective choice. Still, several questions concerning luteal phase support remain unanswered and require further evaluation.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Author contributions
Conceptualization: VZ. Data Curation: MS, MKS, UW, VZ. Formal analysis: MS, TDN, VZ. Methodology: MS, TDN, GM, VZ. Project administration: MS, TDN, VZ. Visualization: MS, TDN, VZ. Writing–original draft: MS, TDN, VZ. Writing–review & editing: all authors.
Acknowledgements
We thank Dr. Brandon Greene (Institute of Medical Biometry and Epidemiology, Philipps University Marburg, Germany) for his support with the statistical analyses.



