Effect of a dual trigger on oocyte maturation in young women with decreased ovarian reserve for the purpose of elective oocyte cryopreservation
Article information
Abstract
Objective
The aim of this study was to determine whether co-administration of a gonadotropin-releasing hormone (GnRH) agonist and human chorionic gonadotropin (hCG) for final oocyte maturation improved mature oocyte cryopreservation outcomes in young women with decreased ovarian reserve (DOR) compared with hCG alone.
Methods
Between January 2016 and August 2019, controlled ovarian stimulation (COS) cycles in women (aged ≤35 years, anti-Müllerian hormone [AMH] <1.2 ng/mL) who underwent elective oocyte cryopreservation for fertility preservation were retrospectively analyzed.
Results
A total of 76 COS cycles were triggered with a GnRH agonist and hCG (the dual group) or hCG alone (the hCG group). The mean age and serum AMH levels were comparable between the two groups. The duration of stimulation, total dose of follicle-stimulating hormone used, and total number of oocytes retrieved were similar. However, the number of mature oocytes retrieved and the oocyte maturation rate were significantly higher in the dual group than in the hCG group (p=0.010 and p<0.001). After controlling for confounders, the dual-trigger method remained a significant factor related to the number of mature oocytes retrieved (p=0.016).
Conclusion
We showed improved mature oocyte collection and maturation rate with the dual triggering of oocyte maturation in young women with DOR. A dual trigger appears to be more beneficial than hCG alone in terms of mature oocyte cryopreservation for young women with DOR.
Introduction
Fertility preservation (FP) techniques consist of oocyte, embryo, and oocyte tissue cryopreservation that can prolong the ability to conceive. The cryopreservation of oocytes matured in vivo that were obtained in controlled ovarian stimulation (COS) cycles has generated many successful results [1,2]. Mature oocyte cryopreservation can now be successfully offered as part of FP programs for patients looking to preserve fertility for medical and social reasons. Thus, this is no longer only an experimental concept [3]. However, the usefulness of cryopreservation of collected immature oocytes before and after in vitro maturation (IVM) is still debatable [4]. Therefore, it is still important for FP to collect a large number of mature oocytes.
Women with decreased ovarian reserve (DOR) are at risk of losing their reproductive ability due to the reduced number and quality of oocytes in the ovary [5,6]. Young women with DOR may be candidates for elective oocyte cryopreservation for FP before ovarian failure. However, previous COS studies were conducted with regards to the in vitro fertilization (IVF) treatment of infertile older women with DOR or young women without DOR (oocyte donors). There is a lack of data on obtaining a large number of mature oocytes in young women with DOR.
Meanwhile, one of the most critical steps during COS is the triggering of final oocyte maturation. Human chorionic gonadotropin (hCG) is usually used as a surrogate for the luteinizing hormone surge for final oocyte maturation, resumption of meiosis, and luteinization of the granulosa cells [7]. To reduce the risk of ovarian hyperstimulation syndrome (OHSS), the substitution of hCG with a gonadotropin-releasing hormone (GnRH) agonist was proposed for IVF high responders [8]. However, adverse effects on clinical pregnancy [9] resulted in the emergence of the concept of a dual trigger with a GnRH agonist along with a low dose of hCG [10]. In addition to IVF high responders, the dual-trigger approach has advantages for normal responders in terms of implantation, oocyte collection yield, and oocyte maturation [11]. Furthermore, the dual-trigger approach has been found to improve the oocyte maturation rate in patients with prior low oocyte maturation rates [12]. These benefits have been attributed to the GnRH agonist induced mid-cycle follicle-stimulating hormone (FSH) surge believed to promote oocyte nuclear maturation and cumulus expansion [13,14]. Results regarding the number of mature oocytes or oocyte maturation rates following a dual trigger compared with hCG alone in poor responders are inconsistent [15,16]. Thus, the aim of this study was to investigate whether a dual trigger with a GnRH agonist along with hCG for final oocyte maturation improved mature oocyte cryopreservation outcomes in young women with DOR compared with hCG alone.
Methods
This study was conducted retrospectively with approval from the Institutional Review Board of CHA Gangnam Medical Center (IRB No. GCI-19-44). Due to the retrospective design, the requirement for informed consent was waived. This retrospective study included women 35 years old and younger who underwent elective oocyte cryopreservation for FP due to DOR. DOR was defined when the serum anti-Müllerian hormone (AMH) level was lower than 1.2 ng/mL at the time of COS initiation [17]. AMH concentrations were measured using an Elecsys AMH immunoassay (Roche Diagnostics, Mannheim, Germany). A total of 122 GnRH antagonist COS cycles performed between January 2016 and August 2019 at a CHA Gangnam Medical Center were selected. The exclusion criteria were women on more than their second cycle, a natural cycle, mild stimulation with oral medication, and retrieval failure. After exclusion, a total of 76 COS cycles were included in the final analysis. The study population was divided into two groups based on the trigger method: a dual trigger with a GnRH agonist and hCG (the dual group, n=40) and a trigger of hCG alone (the hCG group, n=36). Figure 1 depicts the study population. We compared the number of mature oocytes and oocyte maturation rates of the two groups and investigated factors associated with the number of mature oocytes retrieved to determine the effect of each triggering method while controlling for confounding factors.
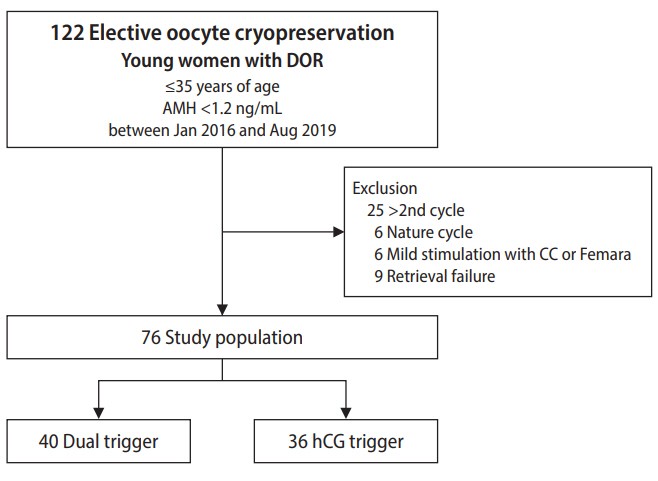
Flow diagram depicting the selection of the study population. DOR, decreased ovarian reserve; AMH, anti-Müllerian hormone; CC, clomiphene citrate; hCG, human chorionic gonadotropin.
Baseline hormone tests and pelvic ultrasound examinations were performed on day 2 or 3 of the menstrual cycle. COS commenced on the 3rd day of the menstrual cycle using recombinant FSH (rFSH; Gonal-F, Merck-Serono, Darmstadt, Germany; Follitrope, LG Life Sciences, Seoul, Korea) and/or highly purified human menopausal gonadotrophin (hMG; IVF-M HP, LG Life Sciences; Menopur, Ferring, Saint-Prex, Switzerland). Patient response was monitored during the COS cycle with serial transvaginal ultrasound examinations, and the gonadotropin dose was adjusted. A GnRH antagonist (Cetrotide, Merck-Serono; Orgalutran, MSD Pharmaceuticals, Courbevoie, France) was added when the leading follicle reached 14 mm in diameter. When at least one of the follicles reached 18 mm in diameter, 250 μg of recombinant hCG (Ovidrel, Merck-Serono) or coadministration of 0.2 mg of a GnRH agonist (Decapeptyl; Ipsen Pharma, Barcelona, Spain) with 250 μg of hCG was administered for final oocyte maturation. The choice of triggering method was made at the physicians’ discretion.
Under transvaginal ultrasound guidance, oocyte retrieval was performed 35 to 36 hours after dual or hCG trigger. Oocyte maturity was evaluated using microscopy, and the mature oocyte count was assessed. Mature oocytes had a single polar body with an expanded cumulus-corona. Immature oocytes were cultured for IVM. The oocyte maturation rate was calculated by dividing the number of mature oocytes by the number of total oocytes. After excluding degenerative oocytes, the oocytes were cryopreserved using the vitrification method.
All statistical analyses were performed using IBM SPSS ver. 25.0 (IBM Corp., Armonk, NY, USA). Continuous variables are presented as mean±standard deviation, and categorical variables are presented as numbers with percentages. Differences between the two groups were analyzed using the Student t-test or the Pearson chi-square test. Furthermore, we used univariate analysis to identify factors related to the number of mature oocytes retrieved. Multivariate analysis was performed to examine the adjusted effect of related factors. Subgroup analysis according to the COS regimen was performed using the Mann-Whitney test, and p-values <0.05 were considered to indicate statistical significance.
Results
The clinical characteristics of the study subjects are summarized in Table 1. No significant differences were observed in age or ovarian reserve (determined by AMH levels and basal FSH). Approximately one-third of the women had a history of ovarian surgery. Table 2 represents the ovarian stimulation and laboratory outcomes. The duration of stimulation, the total dose of FSH, and the estradiol level on the triggering day were similar. The total FSH dose by the number of oocytes retrieved was comparable between the two groups. However, the total FSH dose by the number of mature oocytes retrieved was significantly lower in the dual group than in the hCG group (634 ± 480 IU vs. 982 ± 748 IU, respectively; p=0.030). Although the number of total oocytes retrieved was statistically similar (5.3 ± 3.5 vs. 5.0 ± 2.7, p=0.655), the number of mature oocytes retrieved was significantly higher in the dual group than in the hCG group (3.7 ± 2.7 vs. 2.3 ± 1.7, respectively; p=0.010). Furthermore, the oocyte maturation rate was significantly higher in the dual group than in the hCG group (68.5% vs. 45.6%, respectively; p<0.001). OHSS did not occur in either group.
Univariate correlation analysis was used to determine factors significantly related to the number of mature oocytes retrieved: basal AMH (p=0.041), basal FSH (p=0.014), basal antral follicle count (AFC) (p<0.001), and the dual trigger versus the hCG trigger method (p=0.011). In the multivariate analysis, basal AFC (p<0.001) and the use of the dual trigger method (p=0.016) remained significant factors (Table 3).
Discussion
The present study showed an increased number of mature oocytes retrieved and higher oocyte maturation rates with the use of a dual trigger in young women with DOR undergoing GnRH antagonist-downregulated COS cycles. A dual trigger in elective oocyte cryopreservation for young women with DOR is a viable treatment to cryopreserve a large number of mature oocytes in FP. Single women could achieve reproductive autonomy via elective oocyte cryopreservation. Currently, women with DOR consider elective oocyte cryopreservation before compromising their fertility. Young women with DOR have fewer oocytes retrieved, but the possibility of a high-quality embryo and clinical pregnancy are higher once the oocytes are acquired [18]. Although young women with low AMH levels are challenging subjects for oocyte retrieval due to their poor response to COS [19], they should be counseled about efficient oocyte cryopreservation plans. Efficient oocyte cryopreservation could diminish the pressure on young women to have a child or select a partner at a certain time.
In DOR patients, results regarding the number of mature oocytes following dual trigger compared with a trigger of hCG alone remain inconsistent. Zhang et al. [15] concurred that a dual-trigger approach was beneficial in women with a mean age of 36 years. However, Lin et al. [16] showed no difference in mature oocyte outcomes in women (mean age, 38 years) with DOR undergoing a dual-trigger treatment method. Our study was performed in relatively young women; therefore, a dual-trigger method could be more effective in younger women.
Women undergoing FP want to eventually have a healthy child with their cryopreserved mature oocytes. Although we cannot provide results for embryo quality according to the trigger method, the reproductive outcomes of oocytes treated with a dual-trigger approach do not seem to be compromised. Thorne et al. [20] recently reported that the embryo aneuploidy rate was similar in patients stimulated with a GnRH agonist trigger and patients administered an hCG trigger. Embryo development and quality appear to be unaffected in GnRH agonist trigger cycles [21]. Moreover, a meta-analysis revealed that patients treated with a dual-trigger approach had significantly more good-quality embryos than patients treated with an hCG trigger [22].
The endogenous gonadotropin surge released by the administration of GnRH agonist, similar to the natural cycle surge, is considered more physiological than the hCG trigger. The importance of a mid-cycle FSH surge induced by a GnRH agonist has been determined in several animal and molecular biology studies. Animal studies have confirmed the importance of FSH in the upregulation of LH receptor sites formation in granulosa cells [23,24]. FSH also plays a crucial role in promoting the resumption of oocyte meiosis [25,26] and the expansion of cumulus cells [13,27]. Furthermore, the expression of messenger RNA of reproduction-related genes such as amphiregulin and epiregulin was found to be higher in granulosa cells after a dual-trigger treatment compared to an hCG trigger [28]. Amphiregulin and epiregulin are epidermal growth factor receptor ligands that have been reported to play essential roles in cumulus expansion, oocyte maturation, and meiosis resumption [29,30].
When planning FP, the potential complications and cost of oocyte retrieval should be taken into consideration. In our study, no OHSS or other complications occurred in either group. Although the total dose of FSH used was similar, the efficiency in terms of FSH dose per retrieved mature oocyte was much better with the dual trigger. Further study is needed to evaluate whether the dual-trigger approach is more cost-effective in FP.
We subanalyzed the data according to the gonadotropin combinations (rFSH only or rFSH+hMG; data not shown in the results). The oocyte maturation rates were significantly higher in the dual-trigger group regardless of the COS regimen (rFSH only, 69.5% vs. 45.8%, p<0.001; rFSH with hMG, 67.4% vs. 45.0%, p=0.006). The number of mature oocytes retrieved was higher with the dual-trigger approach, but the difference was not statistically significant due to the small numbers involved (rFSH only: 4.1 ± 2.6 vs. 2.8 ± 1.9, p=0.127; rFSH with hMG: 3.2 ± 2.8 vs. 1.7 ± 1.4, p=0.089). The dual-trigger approach for final oocyte maturation seemed to improve mature oocyte retrieval regardless of the combination of gonadotropins used.
This study had a few limitations, such as the small number of study subjects and the retrospective nature of the study. Moreover, only the result of mature oocyte cryopreservation was reported; the results after cryopreservation of in vitro matured oocytes were not presented. To the best of our knowledge, this is the first study comparing a dual trigger to an hCG-only trigger in elective oocyte cryopreservation for young women with DOR. The beneficial effect of the dual trigger with a GnRH agonist and hCG on obtaining mature oocytes enabled more mature oocyte cryopreservation in young women with DOR. Future prospective randomized controlled studies are required to validate our findings.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Author contributions
Conceptualization: SJK, SWL. Data curation: THK, JKP, JHE. Formal analysis: SJK, THK. Methodology: SJK, WSL, SWL. Project administration: SWL. Visualization: SJK. Writing–original draft: SJK. Writing–review & editing: SJK, WSL, SWL.



