The impact of post-warming culture duration on clinical outcomes of vitrified-warmed single blastocyst transfer cycles
Article information
Abstract
Objective
The objective of the study was to compare the effects of long-term and short-term embryo culture to assess whether there is a correlation between culture duration and clinical outcomes.
Methods
Embryos were divided into two study groups depending on whether their post-warming culture period was long-term (20–24 hours) or short-term (2–4 hours). Embryo morphology was analyzed with a time-lapse monitoring device to estimate the appropriate timing and parameters for evaluating embryos with high implantation potency in both groups. Propensity score matching was performed to adjust the confounding factors across groups. The grades of embryos and blastocoels, morphokinetic parameters, implantation rate, and ongoing pregnancy rate were compared.
Results
No significant differences were observed in the implantation rate or ongoing pregnancy rate between the two groups (long-term culture group vs. short-term culture group: 56.3% vs. 67.9%, p=0.182; 47.3% vs. 53.6%, p=0.513). After warming, there were more expanded and hatching/hatched blastocysts in the long-term culture group than in the short-term culture group, but there was no significant between-group difference in embryo grade. Regarding pregnancy outcomes, the time to complete blastocyst re-expansion after warming is shorter in women who became pregnant than in those who did not in both culture groups (long-term: 2.19±0.63 vs. 4.11±0.81 hours, p=0.003; short-term: 1.17±0.29 vs. 1.94±0.76 hours, p=0.018, respectively).
Conclusion
The outcomes of short-term culture and long-term culture were not significantly different in vitrified-warmed blastocyst transfer. Regardless of the post-warming culture time, the degree of blastocyst re-expansion 3–4 hours after warming is an important marker for embryo selection.
Introduction
Elective single embryo transfer has been widely adopted to reduce the risk of maternal/neonatal complications and multiple births with in vitro fertilization. Accordingly, the need to cryopreserve surplus blastocysts after embryo transfer is increasing. In addition, with the recent trend to take steps to reduce the risk of ovarian hyperstimulation syndrome and to transfer embryos in a more physiologic environment, cryopreservation has become a common strategy. Therefore, interest has been growing in the proper selection criteria for high-quality warmed embryos [1].
In vitrified-warmed blastocyst transfer (VBT), complete blastomere survival and mitotic resumption during warming are generally considered to be the most important factors affecting pregnancy outcomes. It has been assumed that a sufficient warming time may be required for resumption of cell proliferation and development [2]. However, the effect of culture duration on pregnancy outcomes in VBT remains controversial. One study suggested that a short-term culture period of 2–5 hours was associated with more favorable clinical outcomes, although there was no statistically significant difference in mitotic division between the two groups. However, another study showed that the post-warming culture duration (1 hour vs. 18 hours) was not relevant for the implantation rate (IR) and live birth rate when evaluating high-quality vitrified-warmed blastocysts [3,4]. Ebner et al. [5] performed a morphological analysis of the post-warming process of re-expansion and development with a time-lapse monitoring device. The study demonstrated that the completion of re-expansion took 2.70±1.20 hours on average, suggesting that a sufficient time (i.e., not too short) may be needed to evaluate embryo development.
The purpose of the study was to compare the clinical outcomes of warmed blastocysts after long-term culture (20–24 hours) or short-term culture (2–4 hours). The morphological parameters of embryo re-expansion were also analyzed with a time-lapse monitoring device to estimate the appropriate timing and parameters for evaluating embryos and predicting the implantation potency of post-warmed embryos.
Methods
This study was approved by the Institutional Review Board of CHA Gangnam Medical Center (IRB No. GCI-19-27). Patients’ informed consent was not needed due to the nature of retrospective study.
1. Participants
This retrospective study was conducted using the medical records of patients who underwent VBT procedures from March 2017 to December 2018 at the Fertility Center of CHA Gangnam Medical Center. Patients in whom a single blastocyst was transferred, regardless of the number of thawed embryos, were selected for the analysis. Cycles with double blastocysts were excluded due to the uncertainty of the associations with embryo quality when one of the two transferred embryos was implanted. Cycles were excluded if procedural difficulties were encountered or if patients had a thin endometrium (<8 mm). Cycles were divided according to whether the culture period was long-term (20–24 hours) or short-term (2–4 hours).
2. Blastocyst vitrification and warming
For vitrification, blastocysts were first equilibrated in a mixture of HEPES medium (SAGE Quinn’s-HEPES; CooperSurgical, Trumbull, CT, USA) and 20% HSA (SAGE, CooperSurgical) supplemented with 7.5% ethylene glycol (EG) and 7.5% dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO, USA). For the final equilibration, 15% EG, 15% DMSO and 0.5 M sucrose were used. Each blastocyst was loaded onto a gold electron microscopic (EM) grid (EM Grid; SPI Supplies, West Chester, PA, USA). For the warming process, the EM grid containing the blastocyst was sequentially transferred to culture dishes containing HEPES medium and 0.5 M, 0.25 M, 0.125 M, and 0.0 M sucrose at intervals of 2.5 minutes, with 20% human serum albumin (SAGE BioPharma). After warming, the blastocyst was washed with blastocyst medium (Cook Medical, Bloomington, IN, USA) at 37°C in an atmosphere of 6% CO2, 5% O2 and 89% N2 and then cultured.
3. Embryo grading
Blastocyst morphology was evaluated according to the degree of blastocoel expansion and inner cell mass (ICM) and trophectoderm (TE) morphology. The blastocoel expansion grade was categorized into five groups: early, the blastocoel filling <50% of the non-expanded embryo; mid, the blastocoel filling >50% of the embryo; expanded, full blastocyst, cavity completely filling the embryo; hatching, a hatching blastocyst; and hatched, a blastocyst that has completely hatched out of the zona pellucida. ICM morphology was graded as follows: A, many tightly packed cells; B, several loosely grouped cells; and C, very few cells. TE morphology was graded following the same logic: A, many cells creating a cohesive epithelium; B, few cells forming a loose epithelium; and C, very few large cells. Embryo grading was performed using the modified Gardner blastocyst grading system: excellent (E: expanded AA, hatching AA, hatched AA), good (G: early AA, mid AA, expanded AB or BA, hatching AB or BA, hatched AB, BA), average (A: early AB, BB, or BA; mid AB, BA, or BB; expanded BB; hatching BB; hatched BB), and poor (P: early AC, BC, CA, CB, or CC; mid BC, CA, CB, or CC; expanded BC, CB, or CC; hatching BC, CB, or CC; hatched BC, CB, or CC).
4. Time-lapse monitoring of blastocysts after warming
Immediately after warming, blastocysts were cultured in a time-lapse system (Embryoscope; Vitrolife, Göteborg, Sweden). The time-lapse video system captures images at intervals of 10 minutes followed by annotation. For the vitrified-warmed blastocysts, the blastocoel re-expansion time was analyzed in terms of tRE (start of re-expansion) and tCRE (completion of re-expansion), which were recorded and compared between the groups.
5. VBT cycle
All patients underwent endometrial preparation through either hormone replacement therapy or natural cycles. Patients with regular ovulation were treated using natural cycles. Luteal support was started using Crinone vaginal gel 8% (Merck Serono, Geneva, Switzerland) or vaginal Utrogestan (600 mg; Han Hwa Pharmaceuticals, Seoul, Korea) after confirmation of ovulation. In hormone replacement therapy cycles, oral estrogen (6 mg/day) was commenced on the 3rd day of the menstrual cycle until endometrial thickness reached at least 8 mm. Luteal support was applied for 5 days before VBT.
6. Assessed outcome variables
The primary outcomes of the study were clinical pregnancy outcomes, including the IR, clinical pregnancy rate (CPR), and ongoing pregnancy rate (OPR). The IR was calculated as the percentage of embryos that successfully underwent implantation compared to the number of embryos transferred. Clinical pregnancy was defined as the presence of a fetal heartbeat on ultrasonography. An ongoing pregnancy was defined based on a positive fetal heartbeat at 11 weeks or more of gestation confirmed on ultrasonography. Miscarriage was defined as a pregnancy that did not continue until it was classified as an ongoing pregnancy. Any pregnancy where the embryo implanted and developed outside the uterine endometrial cavity was defined as an ectopic pregnancy.
7. Statistical analysis
Logistic regression was used to estimate propensity scores for constructing a propensity score model. Potential confounders to be adjusted were included as variables, such as patient characteristics (female partner’s age, paternal age, body mass index, anti-Müllerian hormone levels, infertility duration, number of previous attempts), fresh cycle variables (intracytoplasmic sperm injection, retrieved oocyte numbers) and endometrial thickness at embryo transfer. For propensity score matching, 2:1 nearest neighbor matching was performed without replacement.
The statistical analysis was performed using the Student t-test or Mann-Whitney U-test for continuous variables and the chi-square test for categoric variables. The level of significance was defined as p<0.05. Baseline characteristics of the groups were presented as the mean±standard deviation or number (percentile). Statistical analysis was done using IBM SPSS ver. 23.0 (IBM Corp., Armonk, NY, USA).
Results
1. Patient characteristics
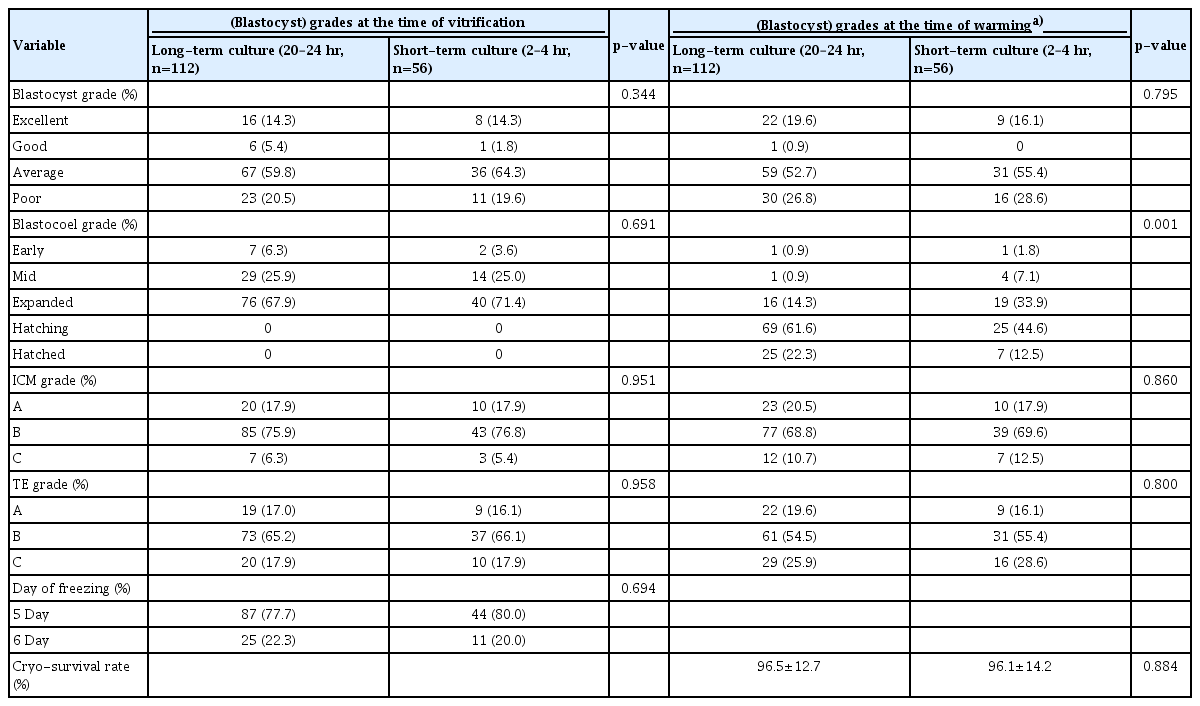
During the study period, a total of 369 single VBT cycles were included: 304 in the long-term group and 65 in the short-term group. After propensity score matching, 108 cycles in the long-term group and 56 in the short-term group were analyzed. Patients’ demographic characteristics did not significantly differ between the groups. An analysis of previous fresh in vitro fertilization cycles and subsequent VBT cycle parameters revealed no differences in the number of retrieved oocytes, the number of fertilized oocytes, and the number of vitrified blastocysts in both groups. Both groups also showed similar characteristics on the day of VBT (Table 1). More embryos were vitrified on day 5 than on day 6, and the cryo-survival rate was 96% (Table 2).
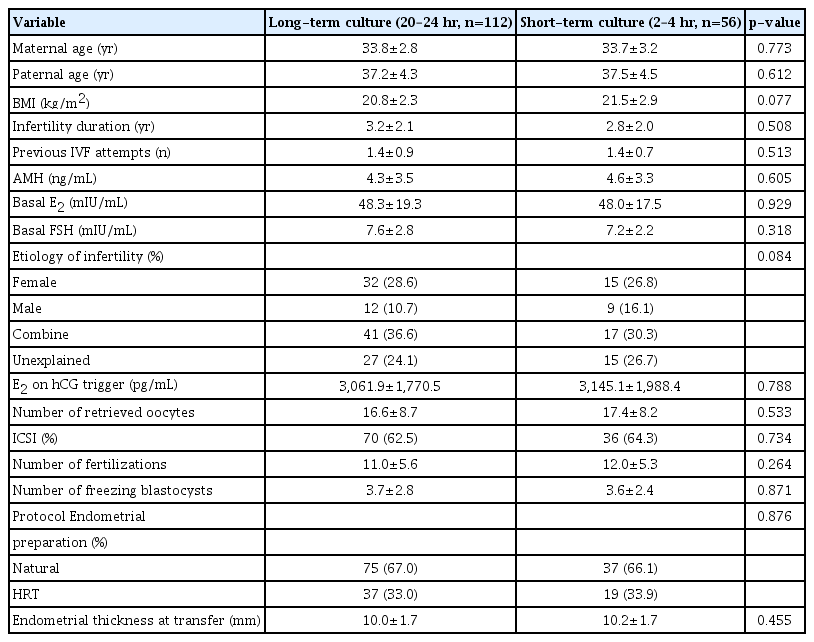
Characteristics of previous fresh IVF cycles and vitrified-warming blastocyst transfer cycle in the two different post-warmed culture period groups
2. Embryo morphological evaluation
The evaluation of the morphological quality of the warmed blastocysts at the time of freezing and at the time of embryo transfer is summarized in Table 2. When evaluating the quality of embryos at the time of vitrification, there were no significant differences in embryo status between the two groups according to blastocyst grade, blastocoel expansion grade, ICM grade, or TE grade. After warming, the embryo quality also remained similar in terms of the grade of blastocysts, ICM and TE. The embryos before vitrification were in the early, mid, and expanded states. However, there was a significant difference between the degree of expansion of the two groups after warming (hatching and hatched embryos, long-term vs. short-term: 83.9% vs. 57.1%, p<0.001).
3. Morphokinetic analysis using a time-lapse monitoring system
The warming embryos were observed through an embryoscope for morphokinetic analysis to evaluate the extent of blastocoel expansion over time. The long-term culture group comprised 58 blastocysts, and the short-term culture group contained 31 blastocysts. There were no embryos that did not start or complete re-expansion during the incubation time in either group.
Regardless of culture duration, the embryos in patients who became pregnant started re-expansion significantly more quickly than embryos in those who did not become pregnant (tRE: long-term group, 0.56 ±0.18 vs. 1.57±0.41, p=0.021; short-term group, 0.37±0.09 vs. 0.81±0.18; p=0.041, respectively) (Figure 1A). tCRE was also faster in patients who became pregnant than in those who did not regardless of culture duration (tCRE: long-term group, 2.19±0.63 vs. 4.11±0.81 hours, p=0.003; short-term group, 1.17±0.29 vs. 1.94±0.76 hours, p=0.018, respectively) (Figure 1B). In patients who became pregnant, the duration for tCRE in the long-term culture group was 0.4–4.7 hours, while that for short-term culture was 0.6–2.7 hours, indicating that all embryos underwent re-expansion within 4 hours.
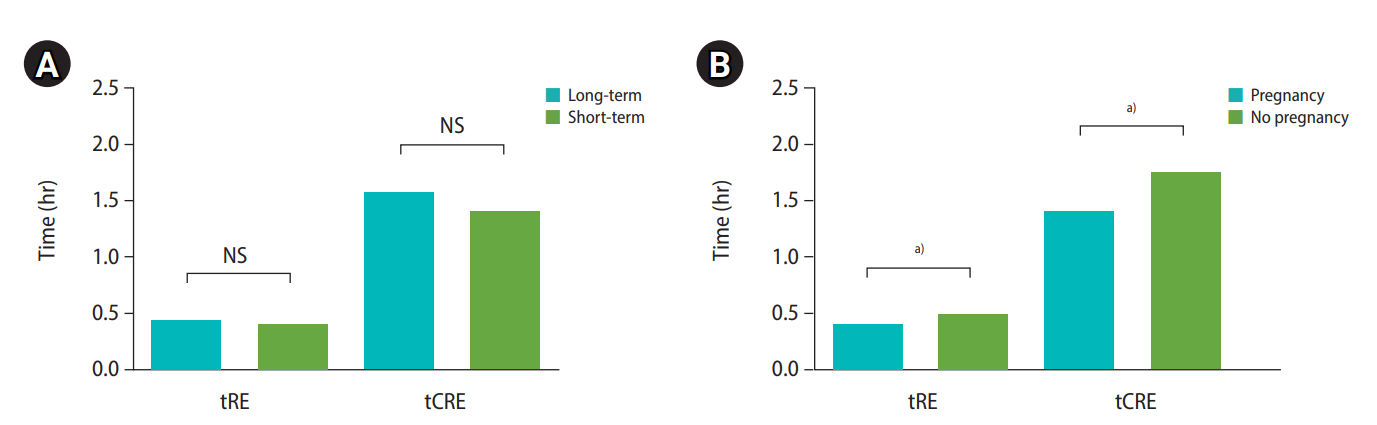
Morphological analysis of blastocoel re-expansion after warming according to ongoing pregnancy in both culture groups with a time-lapse monitoring device. (A) Comparison of the time of the start of re-expansion (tRE) in both culture duration groups. (B) Comparison of the time of the completion of re-expansion (tCRE) in both culture duration groups. Long-term group: 20-24 hours; Short-term group: 2-4 hours; Pregnancy: ongoing pregnancy, defined as the presence of a fetal heartbeat on ultrasonography at 11 weeks or more of gestation. NS, not significant.
a) p<0.05
4. Clinical outcomes
The clinical outcomes of both post-warming culture groups are compared in Table 3. The IR and CPR per embryo transferred were similar in the long-term culture group and the short-term group (56.3% vs. 67.9%, p=0.182 and 53.6% vs. 60.7%, p=0.413, respectively). There was no significant difference in the OPR per embryo transferred between the two groups (47.3% vs. 53.6%, p=0.513). The miscarriage rate in both groups was comparable (p=0.627).
Discussion
The results of this study showed that the clinical outcomes, including the IR, pregnancy rate, and miscarriage rate were similar in the long-term culture group and the short-term culture group. Extended culture time was associated with a higher degree of blastocoel re-expansion just before embryo transfer, but there was no significant difference in the clinical results. Furthermore, through a morphokinetic analysis of vitrified-warmed embryo re-expansion, we demonstrated that the speed of re-expansion was a significant post-warming morphological predictor of clinical pregnancy outcomes in both groups.
The culture time interval after warming may serve as an important factor that affects the quality of the embryo. In the fresh embryo transfer cycles, the implantation potential of embryos has usually been evaluated based on the embryos’ morphology, such as the ICM/TE grade and the degree of blastocoel expansion. However, in VBT cycles, the predictive power of blastocyst morphology is unclear, because the structure of the embryo shrinks through vitrification and then undergoes re-expansion after warming. Some previous reports suggested that long-term culture after warming may increase the degree of blastocoel expansion and increase the chance of selecting an embryo with high implantation potential [3,6]. Du et al. [6] showed that a higher proportion of blastocysts were re-expanded after long-term (20 hours) culture compared with blastocysts with short-term (4 hours) culture (80% vs. 36%), and the IR of embryos cultured for 20 hours was significantly higher.
However, regardless of how the environment of embryo culture aims to mimic Fallopian tube and intrauterine conditions, the influence of stress over culture time always needs to be considered [7]. One study found that reducing the post-thaw culture duration decreased culture-related stress and preserved embryonic developmental potential [8]. Furthermore, as improved vitrification and warming skills have reduced the frequency of embryos undergoing lysis or failing to re-expand, the need for extended culture to deselect blastocysts with the low development potential would have been reduced. Indeed, our study demonstrated that the culture time was not significantly associated with the IR or clinical outcomes. This finding is consistent with results of a previous prospective randomized study showing no association between the culture duration (18 hours vs. 1 hour) and clinical outcomes in the vitrification setting (IR, 38.0% vs. 36.0%; p=0.87) [3].
The proportion of hatching/hatched blastocysts was significantly higher in the long-term culture group (84.8% vs. 53.8%), but all post-warmed embryos were re-expanded at the point of the embryo transfer regardless of the culture period. Ahlstrom et al. [9] assessed cultured embryos after warming for up to 6 hours at short intervals (20–30 seconds), and showed that 2 hours was sufficient to assess the warmed blastocysts. That study suggested that the degree of re-expansion is a direct response after warming, and reported that after 2 hours, time had little effect on the degree of re-expansion [9]. Shu et al. [10] also reported that 75.9% of thawed blastocysts re-expanded after 3 to 4 hours in post-thaw culture. When we analyzed the start and completion time of re-expansion with a time-lapse monitoring device, 90% of embryos started re-expansion immediately after warming and completed re-expansion within an average of 1.4–3.5 hours.
Lin et al. [11] also showed that the blastocyst re-expansion time was not related to the total culture time, but was determined to be a significant indicator of clinical pregnancy outcomes. Other studies also reported that the speed of blastocoel re-expansion during warming significantly predicted clinical outcomes [6,9]. Because of vitrification and cryoprotectant toxicity, less damaged blastocysts with a lower percentage of cell loss tend to undergo an increased degree of re-expansion after warming. In our study, the start time and the completion time of blastocyst re-expansion were found to be significantly faster in patients who became pregnant in both the short-term and long-term culture groups. In the patients who became pregnant, re-expansion began within an average of 0.3–0.5 hours and was completed within 1.1–2.1 hours, approximately twice as fast as in the patients who did not become pregnant. The blastocyst re-expansion mechanism is unclear, but its main process is regulated by the velocity of water flux and resealing of the TE [12]. Under cryodamage, impaired function of Na+/K+-ATPase and water transport mechanisms lead to lesser and slower re-expansion [13]. Therefore, less damaged blastocysts showed faster re-expansion, and the speed of re-expansion may be a promising marker of cellular viability and developmental competency [14].
In order to reduce the potential bias of re-expansion speed on culture time, a subgroup analysis was performed only on embryos that completed re-expansion by 4 hours. This subgroup analysis also showed that the culture time after warming was irrelevant for blastocyst implantation potential, and that the speed of re-expansion was correlated with clinical outcomes, as previously discussed (data not shown). Therefore, long-term culture was considered to have no advantage for selecting embryos with a low implantation potential. At the same time, the extended culture period did not show any detrimental effects on the embryo.
In this study, it was shown the choice of long-term or short-term culture time did not have a significant effect on clinical outcomes. However, the times of start and completion of blastocyst re-expansion can predict the implantation potential, and they showed significance as parameters for selecting appropriate embryos for transfer. Therefore, evaluating the speed of embryo re-expansion 3–4 hours after warming may help to decide which embryo to transfer without waiting until embryos develop to the hatching or hatched stages. This study is limited by its retrospective design and small sample size. Therefore, these findings should be confirmed through prospective randomized controlled studies with larger samples to achieve statistically well-powered results.
In conclusion, the outcomes of short-term culture and long-term culture were not significantly different in VBT cycles. Embryo transfer timing after warming may be determined by optimizing each laboratory’s work flow. Regardless of post-warming culture time, the degree of blastocyst re-expansion 3-4 hours after warming is a valuable marker for embryo selection in warmed embryo transfer cycles.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Author contributions
Conceptualization: SWL. Data curation: JKP, JYH. Formal analysis: JKP, JYH. Methodology & Project administration: THK, JHE, HS, JYK, HMP, CWP, WSL. Visualization: JYH, JKP, SWL. Writing–original draft: JYH, JKP. Writing–review & editing: SWL.