Endometrial profilin 1: a key player in embryo-endometrial crosstalk
Article information
Abstract
Objective
Despite extensive research on implantation failure, little is known about the molecular mechanisms underlying the crosstalk between the embryo and the maternal endometrium, which is critical for successful pregnancy. Profilin 1 (PFN1), which is expressed both in the embryo and in the endometrial epithelium, acts as a potent regulator of actin polymerization and the cytoskeletal network. In this study, we identified the specific role of endometrial PFN1 during embryo implantation.
Methods
Morphological alterations depending on the status of PFN1 expression were assessed in PFN1-depleted or control cells grown on Matrigel-coated cover glass. Day-5 mouse embryos were cocultured with Ishikawa cells. Comparisons of the rates of F-actin formation and embryo attachment were performed by measuring the stability of the attached embryo onto PFN1-depleted or control cells.
Results
Depletion of PFN1 in endometrial epithelial cells induced a significant reduction in cell-cell adhesion displaying less formation of colonies and a more circular cell shape. Mouse embryos co-cultured with PFN1-depleted cells failed to form actin cytoskeletal networks, whereas more F-actin formation in the direction of surrounding PFN1-intact endometrial epithelial cells was detected. Furthermore, significantly lower embryo attachment stability was observed in PFN1-depleted cells than in control cells. This may have been due to reduced endometrial receptivity caused by impaired actin cytoskeletal networks associated with PFN1 deficiency.
Conclusion
These observations definitively demonstrate an important role of PFN1 in mediating cell-cell adhesion during the initial stage of embryo implantation and suggest a potential therapeutic target or novel biomarker for patients suffering from implantation failure.
Introduction
Implantation failure is a major problem in assisted reproductive technology, with a large number of embryos underwent in vitro fertilization (IVF) failing to reach successful pregnancy [1,2]. Prior to embryo implantation, the endometrium undergoes dynamic changes induced by ovarian steroid hormones to produce a period of uterine receptivity referred to as the window of implantation [3-5]. This lasts from day 20 to day 24 of the menstrual cycle in humans [6]. After the endometrium becomes receptive and the embryo reaches the blastocyst stage, effective maternal-conceptus interaction must be initiated for successful implantation [7]. Failure of more than three IVF cycles in which reasonably good embryos were transferred is considered repeated implantation failure (RIF), which occurs in 15%–20% of infertile couples [8]. In patients with RIF, embryo transfer commonly leads to only detection of human chorionic gonadotropin (hCG) or to no detectable status at all, meaning that embryo loss occurs at a very early stage of implantation [9]. Among the factors that attribute to RIF; decreased endometrial receptivity, defective embryos, and unsynchronized maternal-conceptus crosstalk; defects in endometrial receptivity account for approximately one-half of implantation failures in women suffering from RIF after embryo transfer [1,9]. However, little is known about the molecular mechanisms underlying the establishment of endometrial receptivity, in particular regarding the successful initial dialogue between the embryo and the maternal endometrium.
The actin cytoskeleton undergoes highly dynamic structural changes during cell migration, proliferation, and cell-cell adhesion [10]. When the embryo makes initial contact with the maternal endometrium at an early stage of implantation, cytoskeletal remodeling and actin polymerization play a crucial role [11]. It has been previously reported that aberrant actin reorganization impairs the development of mouse embryos produced by IVF during the pre-implantation stage [12]. Members of the profilin family, comprising four isoforms, have been identified as actin binding proteins that are essential for actin polymerization and cytoskeleton organization [13]. Profilin 1 (PFN1) is the most widely-understood protein in the profilin family, and it is reportedly expressed both in the endometrium and in the embryo [14-16]. The loss of PFN1 has been reported to induce the junctional delocalization of E-cadherin with a significant reduction in cell-cell and cell-extracellular matrix adhesion; additionally, it has been shown to elevate cell proliferation in human mammary epithelial cells. In contrast, PFN1 overexpression caused G1 cell cycle arrest as well as the inhibition of cell proliferation and tumor growth in human breast cancer cells [17,18]. However, the specific role of PFN1 in embryo attachment during the initial stage of implantation remains unknown. Therefore, in this study, we first examined the functional roles of PFN1 during the early stage of the embryo-endometrial interaction that mediates embryo attachment during implantation.
Methods
1. Cell culture
Ishikawa cells (a well-differentiated human endometrial adenocarcinoma line) obtained from American Type Cell Culture (Manassas, VA, USA) were maintained in a Dulbecco’s Modified Eagle Medium/F12 medium (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (Gibco), 100 U/mL penicillin (Gibco), 100 mg/mL streptomycin (Gibco), and 2 mM L-glutamine (Gibco). Cells were grown on Matrigel-coated cover glass (1:8 dilution, growth factor-reduced; Corning, Tewksbury, MA, USA) for further attachment assays.
2. Plasmid transfection: lentivirus-mediated RNAi knockdown of PFN1
To knock down PFN1 expression in Ishikawa cells, a lentiviral vector containing shRNA targeting PFN1 (shPFN1) and an empty vector (EV) were constructed with VSVG and d8.9 into L293 cells using the lipofectamine 2000 transfection protocol. The lentiviral vector was administered to Ishikawa cells for 24 hours, and cells were selected with puromycin. PFN1 expression after selection was verified by reversetranscription polymerase chain reaction (RT-PCR) and immunoblotting analyses.
3. Mouse embryo collection and co-culture
All experiments were conducted under a Home Office license, were in compliance with the Animal Act (1986) and had local ethical approval for the care and use of laboratory animals. C57BL/6 strain were maintained in strict accordance with the policies of the CHA University Institutional Animal Care and Use Committee (No. 190126). Female mice (6–8 weeks) were superovulated with 10 IU of pregnant mare serum gonadotropin (Daesung Microbiological Labs, Seoul, Korea), and ovulation was synchronized with 5 IU of hCG (Sigma-Aldrich, St. Louis, MO, USA) 46–48 hours later, with both medications administered by intraperitoneal injection. Females were placed singly with males of the same strain overnight. The presence of a vaginal plug the following morning (day 1 of pregnancy) was used as an indicator of successful mating. Pregnant mice were killed on day 1, 48 hours after hCG injection. One-cell embryos were obtained from the oviduct using a 30-G dissecting needle and a 1-mL syringe to tear open the ampulla of the oviduct and release the embryos. Cumulus cells around the one-cell embryos were washed with 0.1% hyaluronidase at 37°C for 5–10 minutes and dissociated by gentle pipetting with glass pipettes. Collected embryos were washed with M2 medium (Sigma-Aldrich) supplemented with 4 mg/mL bovine serum albumin washed in potassium simplex optimized medium (KSOM; Millipore, Burlington, MA, USA) and cultured in a 20-μL drop of KSOM covered with mineral oil at 5% CO2 and 37°C until the blastocyst stage. Only expanded blastocysts with clearly observable inner cell masses and trophectoderms on day 5 were included in the study.
4. In vitro model for embryo implantation: assessment of the stability of embryo attachment
Day-5 mouse embryos were transferred onto EV- or shPFN1-transduced Ishikawa cells in independent wells of a 24-well plate. Multiple observations of the stability of embryo attachment were performed to identify the distinct stages of attachment between 12 hours and 48 hours of co-culture, as previous reported [19,20]. A standardized plate movement protocol was applied to assess the stability of embryo attachment. The plate was tapped three times laterally and orthogonally to detect unattached embryos. Five stages of attachment were defined and used as a measurement scale, as previously described [19,21].
5. RNA isolation and PCR
Total RNA was extracted from Ishikawa cells using TRIzol reagent (Ambion, Austin, TX, USA). A total of 1 μg of RNA was used to synthesize complementary DNA using superscript IV reverse transcriptase (Invitrogen, Carlsbad, CA, USA) and oligo-dT primer. Using one-tenth of the volume of the complementary DNA, gene expression was quantitatively analyzed using an RT-PCR machine (Bio-Rad, Hercules, CA, USA). Amplification was performed using AccuPower PreMix (Bioneer, Daejeon, Korea) and programmed with 40–45 cycles as follows: denaturation at 95°C for 10 minutes, annealing at 58°C–60°C for 30 seconds, and extension at 72°C for 30 seconds. The PCR products were subjected to electrophoresis using a 2% agarose gel and visualized with G-box software (Syngene, Cambridge, UK). The gene expression levels of the genes of interest were normalized to that of β-actin using ImageJ software.
6. Immunoblotting analysis
Whole lysate protein was extracted from cells using a radioimmunoprecipitation assay buffer with protease and phosphatase inhibitors (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s protocol. In this process, 15 μg of protein was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a 0.2-μm-pore PVDF membrane (Millipore). The membrane was blocked with 5% bovine serum albumin/Tris-Buffered Saline, 0.1% Tween 20 for 1 hour and then incubated with specific primary antibody at 4°C overnight against anti-PFN1 (1:2000; Abcam, Cambridge, UK) and β-actin (1:2000, Abcam), followed by incubation with mouse or rabbit immunoglobulin G-horseradish peroxidase (1:3000, Abcam). Protein bands were visualized using a Lumi Femto solution (Dogen, Seoul, Korea).
7. Immunofluorescence staining
Immunofluorescence staining using antibodies against PFN1 (1:500, Abcam) and phalloidin (1:1000, Abcam) were performed as described previously [22].
8. Statistical analysis
Comparison groups were analyzed with the unpaired Student t-test for parametric distributions. For multiple comparisons, two-way analysis of variance was used, followed by the Sidak multiple comparison test. For all tests, a p-value of < 0.05 was considered to indicate statistical significance (p< 0.05, p< 0.01, p< 0.001, and p< 0.0001).
Results
1. Confirmation of PFN1 knockdown in endometrial epithelial cells
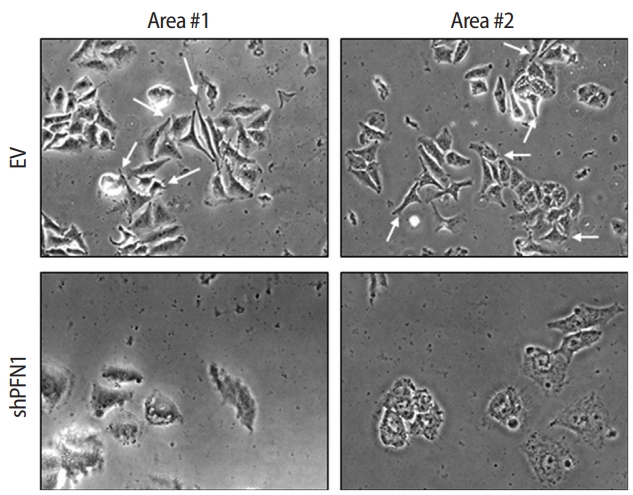
PFN1 acts as a potent regulator of actin cytoskeletal remodeling in response to extracellular signals in epithelial cells [23]. Knockdown of PFN1 expression suppresses fibronectin-promoted cell proliferation and migration in gastric cancer cells [24]. However, the role of PFN1 in endometrial epithelial cells has not yet been examined. To address whether PFN1 in the endometrial epithelium plays an important role in mediating embryonic attachment at the initial stage of implantation, human endometrial epithelial cells (Ishikawa cells) were infected with a lentiviral vector expressing a construct targeting PFN1 shRNA (shPFN1). EV-transduced Ishikawa cells were used as controls across all experiments in this study. Knockdown of PFN1 expression was confirmed by RT-PCR and an immunoblotting assay, demonstrating approximately a six-fold reduction in mRNA and a 2.5-fold decrease in protein levels compared to control-vector-transduced control cells (Figure 1). Morphological examination of Ishikawa cells depending on the status of PFN1 expression revealed that PFN1-depleted Ishikawa cells showed a relatively low extent of spreading and displayed a more circular shape at the edge of cells compared to EVtransduced cells, which is consistent with previous reports showing that the actin cytoskeleton acts as a primary determinant of cell shape and motility [25]. Additionally, more clustering was observed in EV-transduced control cells than in the PFN1-depleted cells (Figure 2). Our data suggest that PFN1 plays a role in mediating cell-cell interactions by regulating the actin cytoskeletal network.
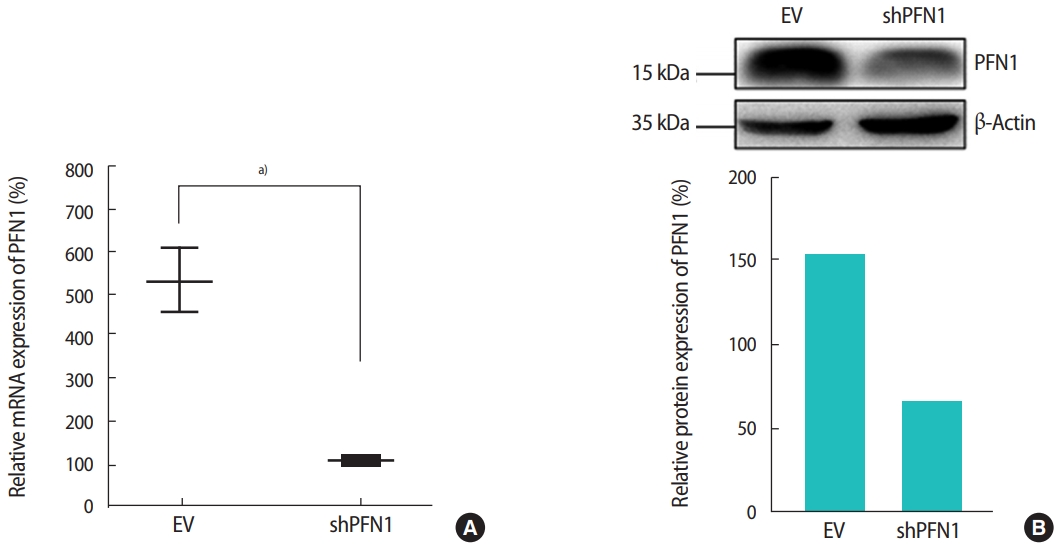
Reverse-transcription polymerase chain reaction (A) and immunoblotting analyses (B) were used to quantify the mRNA and protein expression of profilin 1 (PFN1) in shRNA targeting PFN1 (shPFN1)-transduced Ishikawa cells compared to empty vector (EV)-transduced cells. The densitometry of immunoblot bands shown in (B) was measured using Image J software. a)0.01 <p< 0.05.
2. Knockdown of endometrial PFN1 suppresses the actin cytoskeletal network between the embryo and the endometrium
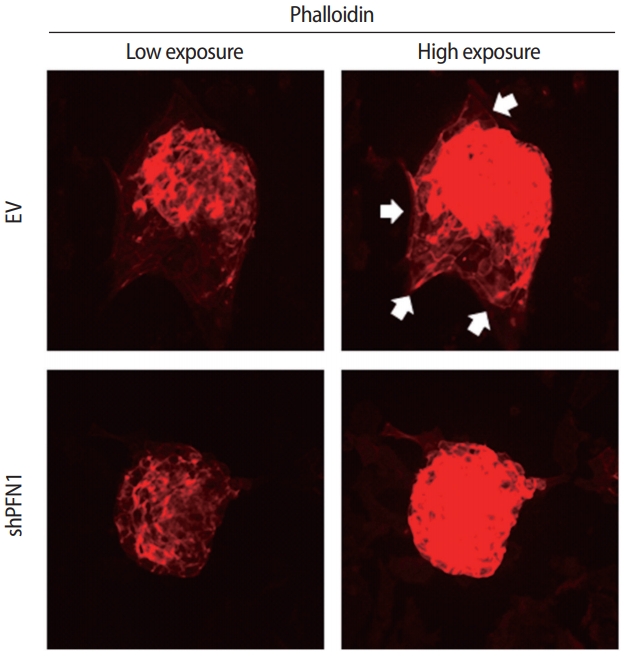
Both cell-cell and cell-extracellular matrix adhesions are facilitated by physical association with the actin cytoskeleton and efficient transmission of the intracellular signaling that promotes cellular adhesion and migration to establish cell-cell interaction [26]. To address whether this is applicable for the initial cell-cell interactions between the embryo and the maternal endometrium, embryonic F-actin formation at the attachment sites on shPFN1-transduced Ishikawa cells compared to EV-transduced control cells was assessed using phalloidin staining. For immunofluorescence analysis, day-5 mouse embryos were co-cultured with shPFN1- or EV-transduced Ishikawa cells. After 48 hours of co-culture, all the embryos used for F-actin formation analyses, were found to be stably attached to both shPFN1- and EV-transduced cells. Embryos attached to EV-transduced Ishikawa cells exhibited more F-actin spreading towards the Ishikawa cells, whereas very little phalloidin expression was detected surrounding the embryos which were attached to shPFN1-transduced cells (Figure 3). Interestingly, all embryos showed significantly higher phalloidin expression than the Ishikawa cells. This may have been due to the difference in depth on microscopy, as embryos in the upper phase express higher levels of phalloidin than Ishikawa cells in the lower phase.
3. Knockdown of PFN1 in endometrial epithelial cells reduces the rate of embryo attachment at the initial stage of implantation
Following the transduction of EV or shPFN1 in Ishikawa cells, a total of 176 5-day-old mouse embryos were transferred (one embryo per well) to confluent cells and were co-cultured for 19, 24, 28, 30, 45, and 48 hours. The stability of the attached mouse embryo was measured according to the five-stage standard: 1, floating; 2, weakly attached but detached after tapping; 3, weakly attached but stuck at the attachment site after tapping; 4, stably attached; and 5, stably attached and showing outgrowth [19,21]. After up to 30 hours of coculture, embryos transferred on control-vector-transduced Ishikawa cells were found to be significantly more stably attached than embryos cocultured with shPFN1-transduced cells, whereas no significant difference was observed at the late stage of co-culture (Figure 4A). Additionally, stably-attached mouse embryos were disturbed by tapping the plates. Interestingly, we found that embryos associated with EV-transduced Ishikawa cells remained more stable than those cocultured with PFN1-depleted cells (Figure 4B). However, after 48 hours of coculture, no significant difference was observed. This might implicate that endometrial PFN1 plays an important role in the early stage of embryo attachment.
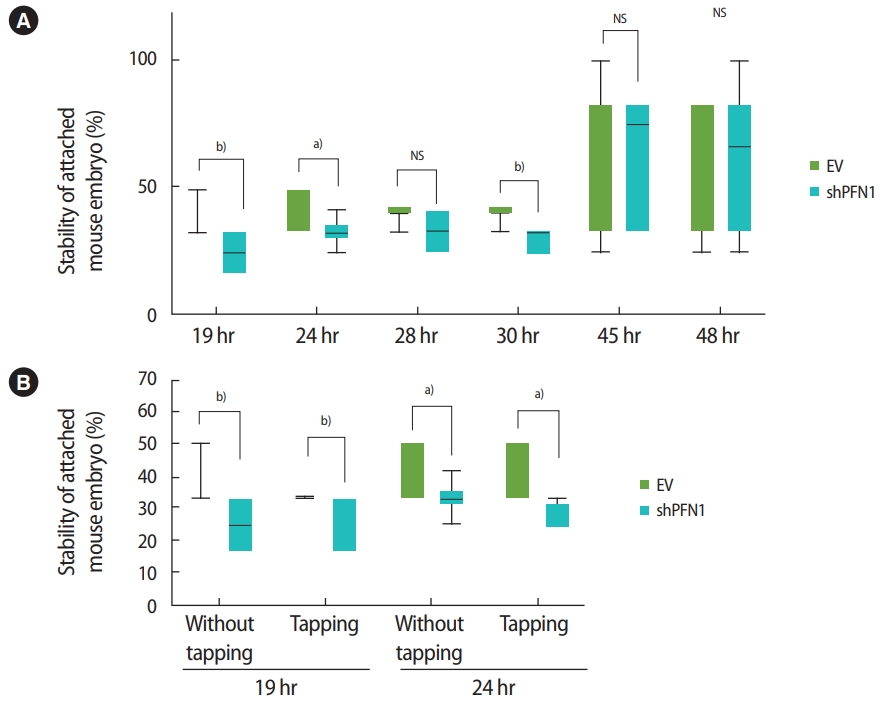
(A) Mean proportions (using 176 day-5 mouse embryos in total) of the stability of embryo attachment after 19, 24, 28, 30, 45, and 48 hours of coculture to empty vector (EV)-transduced or shRNA targeting profilin 1 (shPFN1)-transduced Ishikawa cells. (B) Comparison of mean proportions of the stability of attached embryos at 19 hours and 24 hours with or without disturbance by tapping the plate. Values are presented as mean ± standard error of the mean. NS, not significant. Statistically significant differences: a)0.01 <p< 0.05, b)0.001 <p< 0.01.
Discussion
The data presented in this study revealed the importance of PFN1, a potent regulator of actin polymerization and cytoskeletal remodeling, in facilitating the initial attachment and adhesion between trophoblast cells of the embryo and epithelial cells of the maternal endometrium. This suggests that the PFN1 gene is a critical mediator of the initial stage of embryo implantation. Implantation is historically known to consist of three stages: apposition, adhesion, and invasion [3,27,28]. Both mouse and human embryos exhibit sequential events of embryo attachment beginning with weak initial attachment and subsequent more stable adhesion to the maternal endometrial epithelium [29]. During the attachment stage, appropriate crosstalk between the endometrium and the embryo is crucial for successful implantation, and abnormalities occurring during this process can be the cause of pregnancy failure [3,27,30]. Embryonic loss in patients suffering from RIF often occurs during this early stage of implantation [9]. Despite the extensive research aimed at overcoming implantation failure, especially with regard to embryonic loss at the attachment stage, no clear evidence-based therapeutic strategy currently exists.
PFN1, one of the main regulators of actin dynamics, is a key mediator of cell-cell adhesion in the formation of colonies and the promotion of cell spreading [31,32]. The characteristics observed in endometrial epithelial cells as shown in Figure 2 might be fundamentally applicable to the initial interaction or attachment between the embryo and the maternal endometrial epithelium. Similar to previous findings that showed a significant reduction of F-actin formation with depletion of PFN1 in alveolar epithelial cells [33], our data demonstrated that embryos attached to PFN1-depleted Ishikawa cells displayed reductions in F-actin and stress fiber formation by exhibiting decreased phalloidin expression compared to EV-transduced cells (Figure 3). This may imply that a reduced capacity of cytoskeletal networking between the embryo and the maternal endometrium caused by the absence of endometrial PFN1 impairs endometrial receptivity and decreases the chances of association between these two different types of cells. Additionally, within the cooperative regulation of vinculin and vasodilator-stimulated phosphoprotein (VASP), PFN1 is known to help maintain epithelial cell polarity [34], which is critical for the embryo to initiate attachment onto the maternal epithelium for successful implantation [27]. Depletion of PFN1 might have induced the loss of polarity in Ishikawa cells and subsequently caused the reduction in the rate of embryo implantation at an early point. Furthermore, PFN1 (both nuclear and cytoplasmic) is known to play key roles in oocyte maturation, fertilization, and embryonic development [35]. PFN1 is also detected in serum and conditioned media [36,37]. Although its extracellular function is not well understood, extracellular PFN1 has been reported to be involved in the decidualization of human endometrial stromal cells by acting as an extravillous trophoblast-secreted factor [38], suggesting the importance of a future study examining the impact of extracellular PFN1 on changes in expression at the cellular level in endometrial epithelial cells and improvement of the embryo-endometrial networking environment. However, it was not clear whether proper actin polymerization through intact PFN1 facilitates embryonic attachment to the endometrium. To address this question, we evaluated the role of PFN1 in embryo attachment by measuring the stability of attached embryos at serial time points using a previously-established in vitro model of embryo implantation [19,20]. Our data showed that attachment was delayed in embryos transferred onto PFN1-depleted Ishikawa cells compared to those cocultured with control cells at early points (19 to 30 hours), even though all embryos were stably attached at the late stage of attachment (by 48 hours) (Figure 4). Liang et al. [39] reported that the knockdown of TAGLN2, an actin-binding protein, in trophoblast cells remarkably reduced F-actin formation and impaired trophoblast adhesion and invasion, supporting the importance of actin polymerization in successful embryo implantation, especially during the initial stages.
Overall, this study identified that PFN1 plays an important role in actin cytoskeleton dynamics, which are critical for mediating the cellcell adhesion between the blastocyst and the maternal endometrial epithelium required for successful initial embryo attachment during implantation. Quantification of the stability of the attached embryo revealed that an impaired cytoskeletal environment associated with PFN1 deficiency in the endometrial epithelium significantly reduced the rate of embryo attachment during the early stage of implantation compared to embryos cocultured with PFN1-intact endometrial epithelial cells. Future studies addressing the relevance of the status of endometrial PFN1 with infertility caused by implantation failure may further aid in the identification of new therapeutic targets or novel biomarkers for patients suffering from RIF.
Notes
Conflict of interest
Hwa Seon Koo has been a managing editor of Journal of Clinical and Experimental Reproductive Medicine since 2018; however, she was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Author contributions
Conceptualization: YJK. Data curation: YJK, CJL, SHH, HSK. Formal analysis: YJK, CJL, SHH, KAL, JJK. Funding acquisition: YJK. Methodology: MJY, JHK. Project administration: YJK, DHC, HK. Visualization: CJL, SHH. Writing–original drafting, review & editing: YJK.