Amelioration of lipid abnormalities by vitamin therapy in women using oral contraceptives
Article information
Abstract
Objective
Combined oral contraceptives (COCs) have some adverse effects on the serum lipid profile. Because hyperlipidemia is one of the risk factors in cardiovascular diseases, lipid abnormalities should be evaluated in women consuming COCs. Vitamins E and C are known to have beneficial effects on serum lipid profiles. Therefore, in this study, we evaluated the effects of vitamins E and C on serum lipids in women using COCs.
Methods
The study compared changes in lipid parameters with and without vitamin therapy in women consuming COCs compared to those of a control group (40 non-contraceptive users or NCU) for 4 weeks. Total cholesterol and triglyceride, low-density lipoprotein (LDL), and high-density lipoprotein (HDL) levels along with HDL/LDL ratios were measured for all participants.
Results
COC users experienced significantly higher increases in the levels of triglycerides and LDL than non-users (p<0.05). However, no significant differences were noted in the total cholesterol and HDL levels. In the treated COC group receiving vitamins E and C, the HDL level and the HDL/LDL ratio increased and the LDL and triglycerides levels decreased significantly compared with those of the other groups.
Conclusion
The results of our study indicate that supplementation with antioxidant vitamins E and C restores a normal lipid profile in COC users.
Introduction
In addition to their central role in pregnancy prevention, hormonal contraceptives have direct effects on carbohydrate and lipid metabolism [1,2,3]. Non-contraceptive effects of a combination of hormonal contraceptives have been under study since the early 1960s. Initial investigation has revealed that users of hormonal contraceptives experienced marked impairment of glucose tolerance, impaired insulin secretion, and increases in serum triglycerides and total cholesterol [4,5,6]. Furthermore, combined oral contraceptives (COCs) appear to influence the concentrations of serum lipids and lipoproteins. Since hyperlipidemia is one of the risk factors of coronary artery disease (CAD), investigations of the hyperlipidemic effects of COCs are of utmost importance. The study of the effects of oral contraceptives on the lipid profile is controversial, probably because of the qualitative and quantitative differences among many of the formulations used in drug combinations [7]. Therefore, mixed results have been obtained for the relationship between COC use and the risk of cardiovascular disease [8,9].
Different generations of OCs have been developed mainly to reduce the metabolic and vascular risks of OCs [10]. Therefore, we studied the possible changes in the concentrations of serum lipids and lipoproteins in women using second-generation COCs.
Numerous clinical and epidemiological studies have demonstrated an inverse relationship between high-density lipoproteins (HDL) and the risk of CAD [11]. Inflammation and oxidative stress are pivotal in atherosclerosis, and dietary micronutrients with antioxidant and anti-inflammatory activities may play a critical role in the prevention of CAD [12].
Low-density lipoproteins (LDL) also play a crucial role in atherogenesis, and oxidative modification imparts atherogenicity to LDL [13]. Previous experimental and epidemiological evidence has suggested that some antioxidant vitamins appear to be important in reducing the risk of CAD [14].
It has been shown that lipid peroxidation is involved in the oxidative modification of LDL [15]. Lipid peroxidation starts only after the depletion of natural antioxidants such as vitamin E, vitamin C, and β-carotene in the body [16]. This is supported by the fact that low serum levels of antioxidant vitamins are associated with a high risk of CAD [17]. To the best of our knowledge, there are no reports on the effects of vitamin therapy on the lipid profile of women who are regular COC users.
A previous study has demonstrated that the use of OCs results in an increase in antioxidant defense, particularly involving two glutathione-dependent enzymes. Furthermore, it has also been shown that the use of vitamins E and C reduces the side effects of OCs [18].
The purpose of the present study was to demonstrate the effect of COCs on serum lipid levels in non-obese women and its reversal after vitamin E and C therapy in order to determine whether the changes could be reversed with vitamin therapy.
Methods
1. Study design
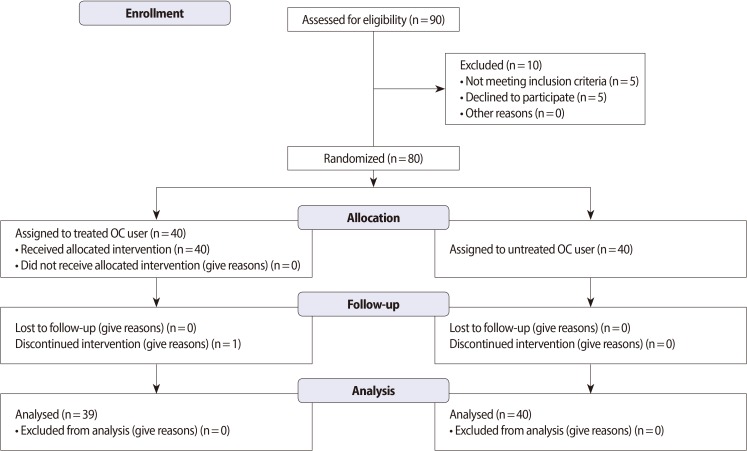
The study design and protocol are shown in Figure 1. The study is a randomized, double-blind, controlled clinical trial with a one-month follow-up period and was conducted at one center in Iran. The research was approved and performed under the guidelines of the Ethics Committee of Shahid Beheshti University Medical Sciences & Health Services and the Iranian Registry of Clinical Trials (Registration No. IRCT201109267641NI). Informed consent was obtained from every participant prior to the initiation of the study.
2. Study participants
Healthy female volunteers within the age range of 18 to 40 years and requesting oral contraceptives were eligible for inclusion in this study. The study population was designed to be representative of the Iranian population. The subjects were women who regularly consulted the health center for their monthly check-up and had a self-reported healthy status, a body mass index (BMI) within the range of 21 to 24 kg/m2, and normal blood pressure. Exclusion criteria included smoking, pregnancy or lactation, a BMI of more than 24 kg/m2 or less than 21 kg/m2, and any disease or condition that could compromise the function of body systems, such as diabetes mellitus, cardiovascular disease, systemic illness with or without secondary hyperlipidemia, medications, antioxidants or vitamin supplements, or alcohol or drug abuse.
3. Study treatments
Approximately 90 individuals that did not show significant differences from the baseline characteristics such as age (p=0.8) were selected as oral contraceptive pills (OCP) users for the vitamin therapy study. However, 10 participants were excluded because they did not satisfy the inclusion criteria. Approximately 80 OCP users were randomly assigned in a double-blind manner into two groups (40 in each group). Forty women who were condom users were selected as the control group. Parameters for determining the appropriate sample size were as follows: α=0.05; zα=1.96; zβ=0.84; and κ=1. The average duration of OC use was 33±26 months. All the oral contraceptive users (OCUs) were taking a contraceptive pill containing 0.03 mg of ethinyl estradiol and 0.15 mg of levonorgestrel. A 1-month randomized and double-blind study design was used, and supplements included vitamin C (250 mg, Zahravi Co., Tehran, Iran) and vitamin E (200 IU, Osveh Co., Tehran, Iran) [19] daily for 4 weeks [20]. Vitamin administration was commenced after obtaining a negative human chorionic gonadotropin pregnancy test result the day after the last administration of the current oral contraception. Women were instructed to take the study medication daily in the morning at approximately the same time of the day, except on the visit day, when they were required to take their study medication at the study center after specimen collection. The treatment group was monitored every week, and if a pill was missed, the subjects were instructed to take the pill as soon as they remembered and to resume their daily dose the next morning (with at least 12 hours between doses).
4. Study assessments
Blood samples were collected beforeh and one month after study initiation. Blood was drawn into ethylenediaminetetraacetic acid-containing tubes and centrifuged within 4 hours to isolate the plasma, which was then immediately frozen and stored at -20℃ until analysis.
5. Measurements
Total cholesterol (TC), triglycerides (TG), HDL, and LDL were measured using kits from Pars Azemoon Company (Tehran, Iran). Briefly, cholesterol or triglycerides were oxidized enzymatically to release hydrogen peroxide, which then interacted with 4-chlorophenol and 4-aminoantipyrene in the presence of peroxidase to yield quinoneimine. Colorimetric quantification of quinoneimine was performed at a wavelength of 546 nm. HDL was measured after precipitating LDL and very low-density lipoprotein (VLDL) and adding phosphotungstic acid in the presence of magnesium ions. LDL was measured after precipitation with heparin at pH 5.04. The HDL/LDL ratio was calculated from the described measurements.
6. Statistical analysis
Statistical analysis was performed using the SPSS ver. 16.0 (SPSS Inc., Chicago, IL, USA), and a p-value of less than 0.05 was considered statistically significant. Continuous variables were expressed as the mean and the standard deviation. Comparisons among different groups were performed through the analysis of variance. The least significant difference post-hoc test was used to determine significant differences among the continuous variables. A paired sample t-test was used to compare the continuous variables within the groups.
Results
This study included three groups: 1) the control group (used condoms as a physical contraceptive device); 2) untreated OCU group; and 3) the treated OCU group. Each group consisted of 40 women. A total of 80 OCU participants were randomized into the two experimental groups. One group included 40 women within the age range of 31.42±5.12 years; they were OCP users but did not receive any vitamin therapy. The other group consisted of40 women within the age range of 30.29±4.6 years; these women were OCP users, received vitamin therapy, and completed the study, except for one participant who discontinued the intervention.
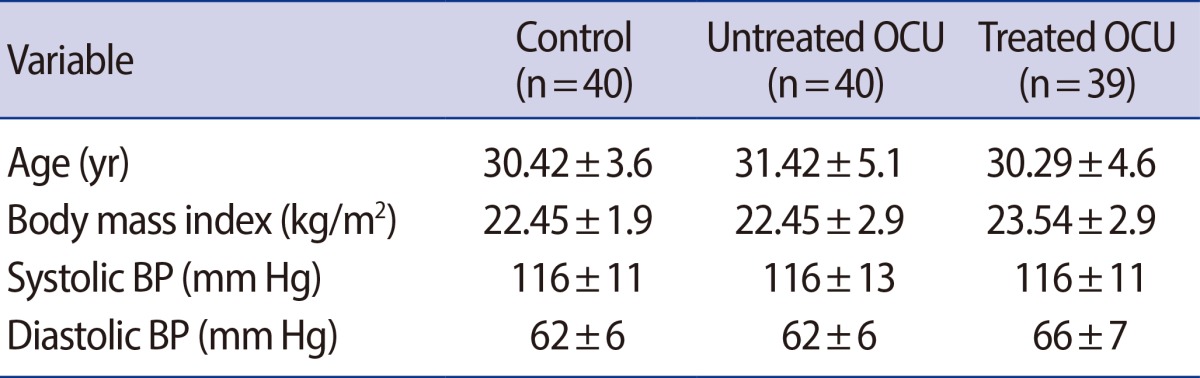
All subjects showed similar baseline characteristics in each phase, as listed in Table 1. No significant differences in BMIs were observed among the groups. No changes in blood pressure were observed among COC users from the untreated and treated groups (Table 1).
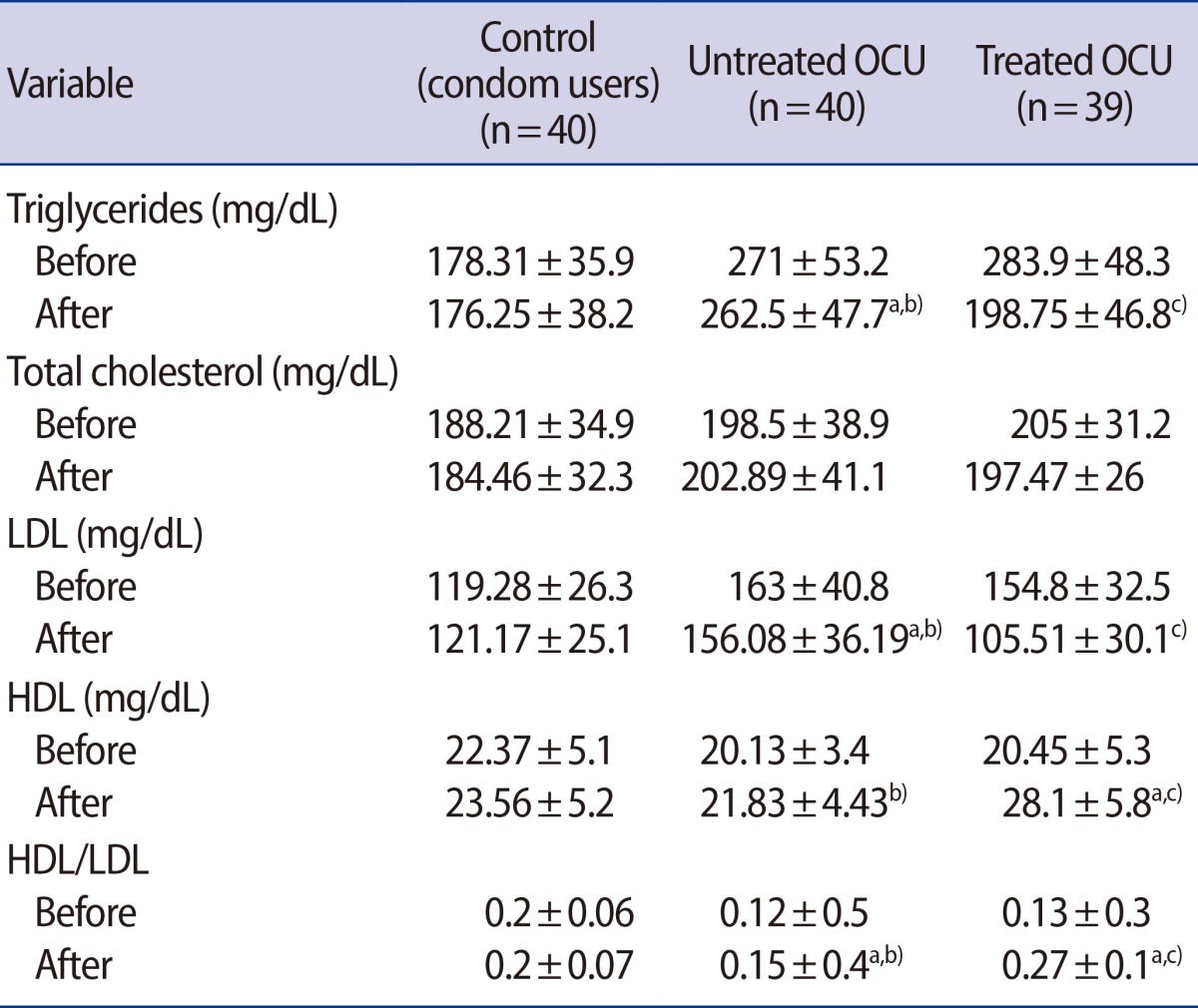
Table 2 shows the changes in lipid profiles, including those in the TC, TG, HDL, and LDL levels and in the HDL/LDL ratio, among the three groups before and after intervention.
Table 2 shows that the plasma TG concentration was 49% higher in the untreated OCU group than in the control group (p<0.05) and significantly decreased in the treated OCU group (p<0.05). The TG concentrations of the treated OCU group showed a 29% decrease after 4 weeks of treatment (p=0.02). The TC levels of the untreated OCU group were slightly but not significantly higher than those of the control and the treated OCU groups. The LDL levels of the treated OCU group were not significantly lower than those of the control group (Table 2). However, the treated OCU group showed a 32% reduction in LDL levels as compared to the untreated OCU group (p<0.05). No changes in the HDL levels were observed after OCP consumption, although this was associated with a 25% decrease in the HDL/LDL ratio as compared to the control group. However, with vitamin E and C therapy, these levels were increased by 28% and 80%, respectively, in the treated group as compared to the untreated OCU group (p<0.05) (Table 2)
Discussion
COCs have an estrogen and a progestogen component. Existing progestogen compounds can be classified as first-, second-, and third-generation compounds. First-generation progestogens include, among others, norethisterone, norethindrone, ethynodiol diacetate, and lynestrenol. Second-generation progestogens include levonorgestrel and norgestrel, and third-generation progestogens include desogestrel, gestodene, and norgestimate [21]. A comprehensive review of several studies shows that TC changes resulting from the use of COCs were minimal [22]. We also did not observe any significant changes in the TC levels among second-generation OC users, which was consistent with previous reports involving third-generation OCs. However, we detected a 49% increase in plasma TG levels in the untreated OCU group. This was consistent with a previous report that demonstrated that ethinyl estradiol increased the secretion of TG by the liver [23]. This result is not of great concern because it has earlier been shown that a rise in the level of estrogen-induced TG does not increase the risk of coronary heart disease when accompanied by an elevated high density lipoprotein-cholesterol (HDL-C) level [24].
COC consumption resulted in a 22% increase in the LDL level, although the HDL level remained unchanged and the HDL/LDL ratio markedly decreased relative to that of the control group.
Sissan and Leelamma [25] showed that the increases in LDL concentrations in female rats receiving 17 beta-estradiol were associated with concomitant decreases in the plasma HDL level.
Duvillard et al. [26] reported that OC-containing ethinyl estradiol plus gestodene induced an increase in the production rate of apoB-containing lipoproteins such as VLDL, intermediate-density lipoprotein, and LDL.
The effects of OCs on plasma HDL concentrations in women have been confusing due to the use of different types and combinations of different doses of estrogens and progestogens in the studies performed. Decreased plasma HDL concentrations have also been reported in OC users [27]. Berenson et al. [28] showed that the use of a very low dose of OC containing desogestrel could elevate lipid levels. They also demonstrated that injectable contraceptive users are at an increased risk of developing an abnormally low plasma HDL-C level as well as an abnormally high LDL level together with an increased LDL/HDL cholesterol ratio, although these effects appeared to be temporary.
The estrogen component of OCs could be responsible for the lipid alterations. However, in terms of triglyceride metabolism, these changes in lipid levels may result in a biochemical defect. The actual defect that is responsible for these alterations in patients using OCs remains elusive.
In this investigation, vitamin therapy not only significantly decreased the plasma LDL levels but also increased the HDL levels and the HDL/LDL ratios.
Multivitamins and mineral supplements have been shown to have beneficial effects on lipid profiles [29]. In a study with human subjects, vitamin C supplementation increased plasma lipid-standardized α-tocopherol [30]. Iribhogbe et al. [31] concluded that the synergetic potential of antioxidant vitamin combinations appears to be beneficial in the management of pregnancy-related hyperlipidemic states.
This study examined the effectiveness of vitamins C and E in regenerating oxidized vitamin E in its active form. The key step is the reaction between the tocopheroxyl radical and vitamin C. Vitamin C regenerates active vitamin E and increases cholesterol excretion [32]. Although this study has one limitation in that it was conducted for a short duration, the administration of a vitamin combination therapy resulted in a significant increase in HDL levels and HDL/LDL ratios.
The results of this study thus suggest that an antioxidant-vitamin combination could be synergistically beneficial to managing OC-related hyperlipidemic states.
These experiments suggest that one has to be cautious in prescribing COCs to patients who already have elevated lipid levels, and all physicians should be alert to the possibility of the emergence of some problems secondary to hyperlipidemia in patients receiving COCs. It is advisable to have an estimate of fasting plasma TG and TC levels before prescribing COCs. If abnormalities are found, other types of birth control methods should be advised. Whenever contraceptive steroids are utilized, only the lowest permissible dose of estrogens should be used and a close follow-up with a periodic measurement of plasma lipid levels is needed. However, taking vitamins E and C may also be beneficial in ameliorating the side effects of the OCs; supplementation with 150 mg of vitamin C and 200 IU of vitamin E in addition to a normal diet may help in improving the plasma lipid profiles in women consuming COCs.
Notes
No potential conflict of interest relevant to this article was reported.