Effect of thymectomy on the female reproductive cycle in neonatal guinea pigs
Article information
Abstract
Objective
The appropriate function of the hypothalamic-pituitary-gonadal axis is essential for maintaining proper reproductive function. In female mammals, the hypothalamic-pituitary-gonadal axis regulates reproductive changes that take place in the estrus cycle and are necessary for successful reproduction. This study was conducted to investigate the effect of thymectomy on the estrus cycle in neonatally thymectomized guinea pigs.
Methods
In this study, 12 female guinea pigs, six thymectomized and six sham-operated, were studied. The effects of neonatal thymectomy at 5–7 days of age on parameters of the reproductive axis were examined in female guinea pigs. Gonadotropin and 17β-estradiol levels were assessed at regular intervals (days 0, 3, 6, 9, 12, and 15) of the estrus cycle, and the time of vaginal opening in the thymectomized and shamoperated guinea pigs was determined.
Results
Significant reductions in gonadotropins and 17β-estradiol levels during estrus cycle were found in neonatally thymectomized female guinea pigs compared to sham-operated guinea pigs.
Conclusion
The results of this study underscore the importance of the thymus in the neonatal period for normal female reproductive function.
Introduction
The hypothalamic–pituitary–gonadal axis, which regulates the reproductive changes that take place during the estrous cycle, is critical to reproductive success [1-3]. In guinea pigs, specific hormones drive the changes that occur during each phase of the cycle and induce anatomical transformations [4]. The hypothalamic–pituitary–gonadal axis originates in the hypothalamus, which serves as an integration center through the release of gonadotropin releasing hormone; this hormone acts on the anterior pituitary and stimulates the synthesis and secretion of gonadotropins. Changes in the contractility and development of the reproductive tract are regulated by cyclic alterations in the secretory patterns of steroid hormones. In females, the gonadotropic hormone follicle-stimulating hormone (FSH) promotes follicular maturation and estrogen synthesis, while luteinizing hormone (LH) acts on the ovarian follicles to promote ovulation and development of the corpus luteum [5-8]. Estrogen is a principal reproductive hormone that influences development and growth, maintaining the properties of the genital tract, as well as sexual behavior and drive [9,10]. Rodents normally produce three fundamental types of estrogen: 17β-estradiol, estrone, and estriol. Estrone and estradiol are synthesized from testosterone and androstenedione in processes catalysed by aromatase [11]. Estradiol induces the differentiation of granulosa cells in the follicle and serves to promote the activity of FSH and LH [12]. An increase in estrogen levels is prerequisite for the LH surge, which promotes ovulation, luteinization of the follicular cells of the ovary, and the synthesis of the hormone progesterone. The consecutive discharge of estradiol and progesterone acts simultaneously on the reproductive neurocircuits and the organs of the genital tract to coordinate reproductive behavior and facilitates the readiness of the uterine endometrium to ensure implantation of the embryo [13,14].
The presence of the thymus during early life is essential for the normal development of the immune system, as well as the proper maturation of the hypothalamic–pituitary–gonadal axis [15]. Previous studies have reported this axis a primarily playing a role in the proper function and maturation of the reproductive system, for which is essential [16,17]. The functional relationship between the thymus and the gonads was first described by Calzolari in 1898 [18]. In other study, thymectomized female rats exhibited a decline in hypothalamic LH-releasing hormone (LHRH) and plasma estradiol levels [19]. Multiple authors have also reported that the proper function of the hypothalamic–pituitary–gonadal axis is dependent on thymic endocrine influences [20,21], but the effect of thymic hormones on target endocrine glands of the hypothalamic–pituitary–gonadal axis has not been sufficiently studied. The functional relationship between the nervous and endocrine systems thus constitutes an integrated homeostatic network within this network, and the interaction between the thymic endocrine and the reproductive systems appears particularly significant. A number of researchers have hypothesized that the thymus may be involved in the developmental programming of the neuroendocrine function of the hypothalamus. Thymic epithelial cells produce hormones such as non-peptide thymulin and thymosin, which enhance the release of LH and LHRH from the anterior part of the pituitary and influence hormone production by the gonads [22,23]. Thymosin fraction 5 advances vaginal opening and elevates estrogen levels in female mice. Production of thymulin and thymosin β1 is inhibited by treatment with sex hormones, and ovariectomy has been shown to increase thymus weight in mice [24,25].
Congenitally athymic female mice have been observed to show delayed vaginal opening due to low levels of estradiol [26], and in a separate study, prepubertal athymic female mice exhibited a decline in the pituitary concentrations of gonadotropins compared to the control animals [27]. Implantation of thymic tissue into nude and thymectomized mice prevents these effects [28,29]. Neonatal thymectomized mice had lower level of FSH, growth hormone, estradiol and progesterone and the effect of thymectomy on the ovary may involve the disruption of the hypothalamic–pituitary–ovarian–thymic axis, which does not appear to involve the immune activity associated with auto immune ovarian dysgenesis [30]. Together, these results show that the thymus can influence the development and proper function of the female reproductive system. To our knowledge, this is the first study of the effect of thymectomy on hormonal changes during the estrous cycle in neonatally thymectomized guinea pigs. In the present experiment, the additional element of a suggested thymus-reproductive system interactions was examined.
Methods
1. Experimental animal
Experimental studies were performed on female guinea pigs who were thymectomized at 1 week old (neonatal average weight, 90 to 100 g). In this study, a total of 12 female guinea pigs (n=12) were studied. The first group (n=6) constituted the sham-operated (Shax) group, in which animals underwent the full operating procedure, but without ablation of thymus gland. The second group (n=6) constituted the thymectomy (Tx) group, which consisted of guinea pigs that underwent surgical removal of the thymus. The animals were purchased from the Institute of Experimental Animals, located in Bangalore, Karnataka, India. The guinea pigs were housed in the SRM Central Animal House and kept in pathogen-free stainless-steel pans with corn-cob bedding material. Four animals were held in each cage, with an autoclaved pots and disposable cardboard boxes provided for hiding, the latter of which are thought to potentially prevent aggressive behavior in animals. Double-distilled water, pellets, and fruits were provided at regular intervals, and the environment was maintained at 60%–65% humidity. The room was maintained with adequate lighting consisting of an alternating light/dark cycle for 12 hours each per/day, and the temperature was held constant at 25°C±2°C. The experimental protocols were approved by the Institutional Animal Ethical Committee (No. 16111/835re-S-04/IAEC2016) of SRM Institute of Science and Technology in Chennai, in accordance with the guidelines set forth by the Committee for the Purpose of Control and Supervision of Experiments on Animals.
2. Thymectomy
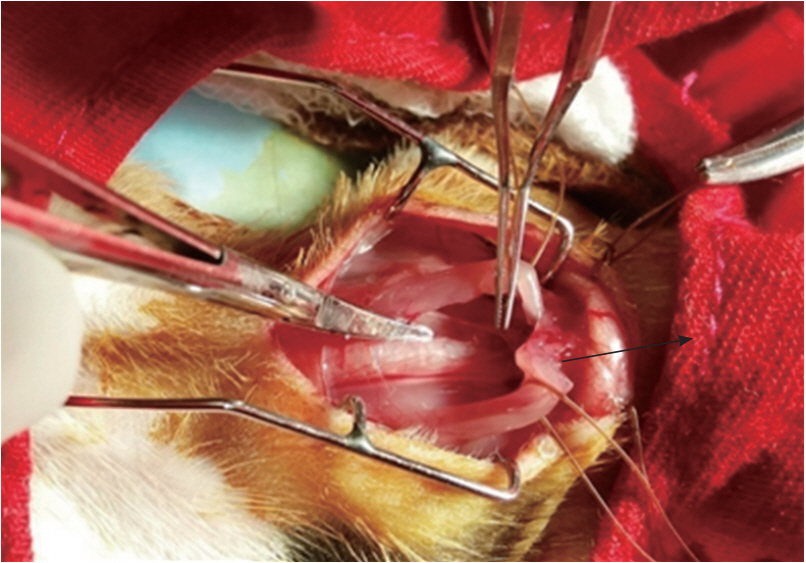
In this study, animals were anesthetized in an isoflurane induction chamber, in which the guinea pigs were sedated with vaporized 3% isoflurane-O2 (3 L/min). Each animal was monitored until it entered the recumbent position, at which time the animal was taken from the induction chamber, its head was positioned with the aid of adhesive tape, and it was placed in the nose cone for ventilation and anesthetic purposes. The operative animals were placed in the supine position upon an operating table with the head pointed towards the operator. The legs were secured to the operating surface with the help of four bands of plastic adhesive strapping. The neck and upper part of the thorax were arched using a small cotton pillow roll beneath the cervical region, which helps to attain the complete exposure of the superior mediastinum and thymus (Figure 1). In this study, thymectomy of the guinea pigs was performed according to the procedure described by Adams [31]. The Shax group of guinea pigs, underwent the same surgical procedures as the Tx group, apart from the removal of the thymus gland. After the procedures, the animals were allowed to recover under a heat lamp, after which they were wiped of their blood stains and cleaned according to standardized aseptic methods. After surgery, the guinea pigs were kept in special cages with soft bedding material.
3. Hormonal assay
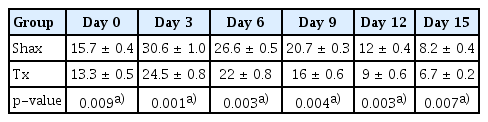
Blood samples were collected to test for gonadotropins and 17β-estradiol on day 0 and at 3 day intervals during the estrous cycle, for a total of 15 days [32]. FSH and LH levels were estimated using the enzyme-linked immunosorbent assay (ELISA) method. These assays were intended to recognize both the natural and recombinant forms of their targets. For both FSH and LH, 1.5-hour solid-phase ELISA kits were used (BlueGene Biotech, Shanghai, China). LH and FSH were added to the wells, which had been precoated with LH and FSH monoclonal antibodies. After incubation, a biotin-conjugated antiguinea pig LH and FSH antibodies were added so that they would bind to guinea pig LH and FSH. After another round of incubation, the unbound biotin-conjugated antibodies were washed away. Streptavidin-horseradish peroxidase, which binds to the biotin-conjugated anti-guinea pig LH and FSH antibodies was added. After incubation unbound streptavidin-horseradish peroxidase was washed away. A substrate solution was added, and color developed in proportion to the amount of guinea pig LH and FSH. The reactions were terminated by the addition of an acidic stop solution, and the absorbance was measured at 450 nm. The sensitivity of the assay was 1.0 ng/mL. Estradiol was measured by radioimmunoassay using the solid- phase iodine 125 technique, with a Coat-A-Count kits (Diagnostic Products Corp., Los Angeles, CA, USA), following the method described by Beckman. Serum gonadotropin and 17β-estradiol hormonal assays were analyzed at several points (days 0, 3, 6, 9, 12, and 15) during the estrus cycle in both groups [32].
4. Vaginal opening and length of estrous cycle
Examination of the vaginal opening was performed in the neonatal female guinea pigs by daily visual inspection of the vagina. After observation of the vaginal opening, the guinea pig was kept in a separate cage to detect the length of estrous cycle by vaginal cytology [33]. Stages of the cycle were assigned using the following criteria, as described in previous studies: proestrus, predominantly nucleated epithelial cells with leukocytes absent; estrous, sheets of non-nucleated squamous cornified cells with leukocytes absent; metestrus, equal distribution of leukocytes and cornified and nucleated epithelial cells; and diestrus, a mixture of epithelial cells and leukocytes, with leukocytes predominating (Figure 2). Vaginal smears were collected daily using swabs of sterile cotton buds. The swab was gently turned (clockwise and counter clockwise) and rolled against the vaginal wall, and then removed. After withdrawal of the cotton swab, the tip was rolled (not slide or rubbed) along the length of a glass microscope slide. Generally, two parallel tracks can be rolled on a single slide and can be immediately fixed in absolute alcohol for staining purposes. The slides were then air-dried followed by Giemsa staining and the cytology was evaluated under a light microscope at ×5 and ×10 magnifications. Day 0 was determined as the day when the vagina of the guinea pig opened completely (in other words, complete absence of the vaginal membrane) [34].
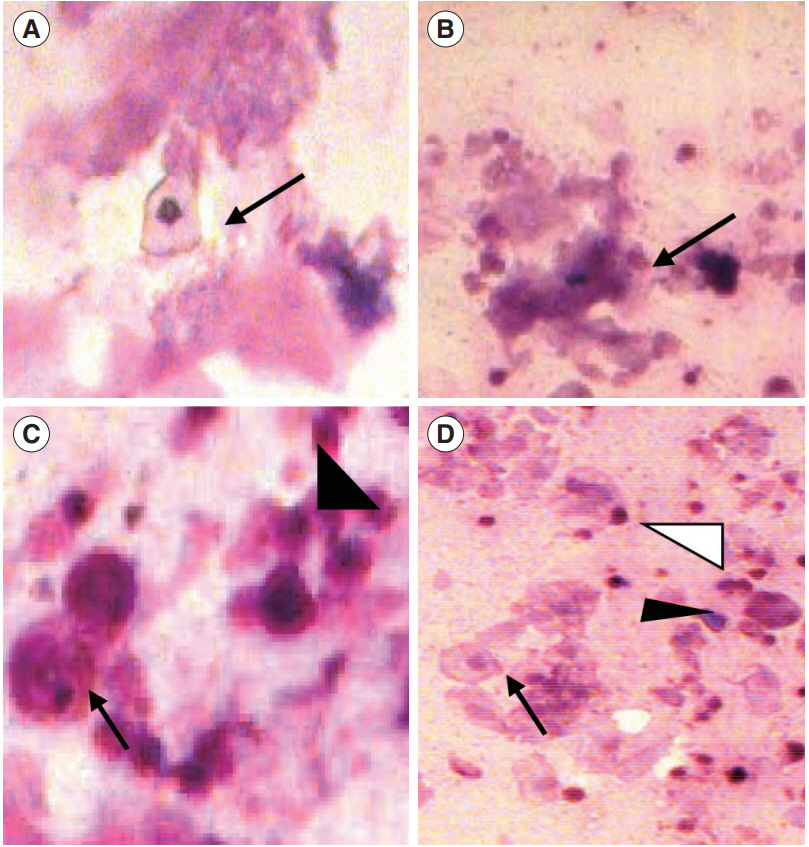
Detection of the estrous phase. (A) Proestrus-enucleated superficial cell (arrow). (B) Estrus-cornified epithelial cells (arrow). (C) Metestrus-parabasal cell (arrow) and neutrophils (arrowhead). (D) Diestrus-parabasal cell (arrow), neutrophils (white arrowhead), and small intermediate cells (black arrowhead). Giemsa staining; magnification, ×5 and ×10.
5. Data analysis
Descriptive statistics were expressed as mean values, standard error of the mean, and range. Gonadotropins and 17β-estradiol were analyzed using the t-test to compare the difference between the two groups (using the Kruskal-Wallis test or one-way analysis of variance). The Statistical analysis was performed using GraphPad Prism 6 software (GraphpPad, San Diego, CA, USA), and p<0.05 were considered to indicate statistical significance.
Results
1. Mortality
The mortality rate of both the Shax and Tx groups were <10%. Death of the animals during surgery occurred due to accidental rupture of the jugular vein.
2. Length of estrous cycle
The length of the estrous cycle in thymectomized neonatal female guinea pigs may vary, but the following average values were determined: proestrus, 3±0.8 days; estrus, 1±0.2 days; metestrus, 6±2.1 days; diestrus, 6±1.5 days, for a total of 16±2.5 days. No significant difference in the length of the estrous cycle could be discerned between the thymectomized (17±0.5 days) and sham operated (16±1.5 days) animals.
3. Day of vaginal opening
Vaginal opening occurred in the neonatal female guinea pig at a mean of 37±1.0 days of age in the thymectomized animals (n=6) and at a mean of 34±0.5 days of age in the sham-operated animals (n=6). The difference between the groups was statistically significant.
4. Serum gonadotropin and 17β-estradiol during the estrous cycle
The mean levels of FSH, LH, and 17β-estradiol were analyzed by one-way analysis of variance between the Shax and Tx groups. The serum FSH level was statistically significantly different between the sham-operated and experimental animals at days 0, 6, and 9 of the estrus cycle. However, there were no significant difference at days 3, 12, and 15 of the estrous cycle between the sham-operated and thymectomized neonatal guinea pigs (Table 1, Figure 3). Serum levels of LH and 17β-estradiol in the thymectomized animals were significantly different from those in the sham-operated guinea pigs (Tables 2 and 3, Figures 4 and 5).

Follicle-stimulating hormone assay of neonatal female guinea pigs analyzed in estrous cycle follow-up
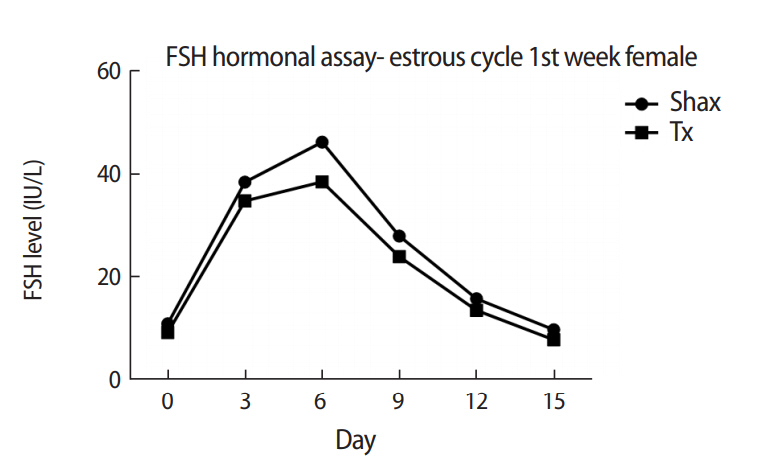
Comparison of serum levels of follicle-stimulating hormone (FSH) in neonatal female guinea pigs at different points (days 0, 3, 6, 9, 12, and 15) of the estrous cycle between the sham-operated (Shax) and thymectomized (Tx) groups.
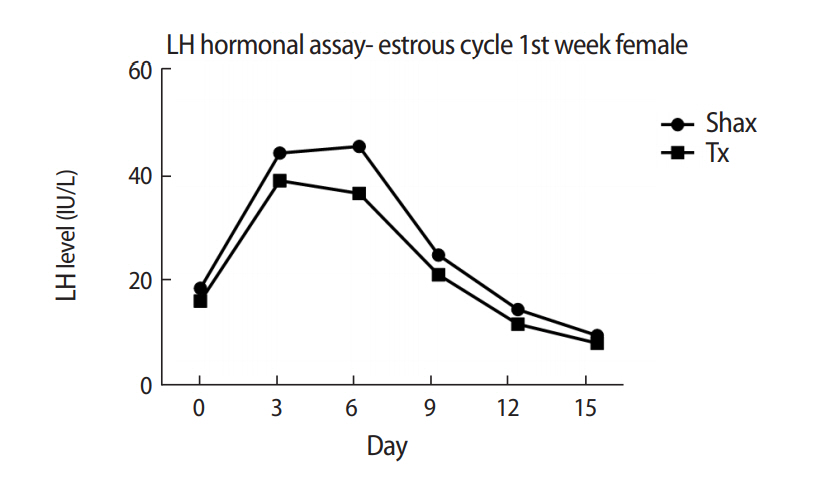
Comparison of serum levels of luteinizing hormone (LH) in neonatal female guinea pigs at different points (days 0, 3, 6, 9, 12, and 15) of the estrous cycle between sham-operated (Shax) and thymectomized (Tx) groups.
Discussion
In 1992, Itoh et al. [35] found that the hormonal impact of thymectomy contributes to morphological changes in the gonads in mice. Opening of the vaginal membrane precedes estrus and allows for accurate establishment of the onset of estrus [36]. The present study was designed to assess the effect of thymectomy on the estrous cycle in neonatally thymectomized guinea pigs. This study showed a delayed onset of vaginal opening in the thymectomized guinea pigs compared to the sham-operated animals. In contrast, no significant changes were observed in the length of the estrous cycle between thymectomized and sham-operated neonatal guinea pigs. However, slight variations were noted in the proestrus and estrus phase of the estrous cycle in two of the six neonatally thymectomized animals. This observation is consistent with previous findings that athymic female mice show a delay in vaginal opening compared with control animals due to reduced levels of gonadotropins and estradiol [37,38]. In another study, mice thymectomized at 10 days old showed a delayed onset of puberty that could be explained by their lower levels of 17β-estradiol, and injection of thymulin resulted in normal onset of puberty and serum estradiol concentration [26]. In 1980, Michael et al. [39] observed that thymectomized mice displayed relatively low serum levels of LH and FSH during the postnatal growth stage. These results propose that developmental reproductive failure in congenitally athymic mice is due to decreased or absent prepubertal gonadotropin priming [39]. In contrast, a study of neonatally thymectomized female mice showed that the alteration of gonadotropins is not sufficient to change the time of vaginal opening and initiation of reproductive cyclicity [40]. The low level of gonadotropins between 9 and 20 days of age in mice thymectomized at 3 days old in a separate study suggested that the gonadotropin levels were adequate for normal ovarian development via an as-yet-unexplained thymus–pituitary–ovarian axis [41].
Additionally, Pierpaoli and Besedovsky [42] reported that the connection between the immunological system and the thymus in adult rodents led to significantly increased serum LH and FSH levels in response to the infusion of allogenic lymphatic cells. Rebar et al. [43] observed that the administration of thymosin fraction 5 and thymosin β4 induced an increase in gonadotropin-releasing hormone from the pituitary gland using a hypothalamic-pituitary perfused system. Goya et al. [44] reported that the immunoneutralization of circulating molecules specifically synthesized by the thymus can increase the gonadotropin deficiencies caused by thymectomy during the neonatal period or by congenital absence of the thymus in mice. Those results show that the lack of thymulin during the initial 8 days of life is sufficient to induce low serum levels of gonadotropins and morphological changes at the adeno-hypophysis of the pituitary gland in adult animals.
Guinea pigs are poly estrous animals, ovulate spontaneously, and have a mean estrous cycle length of 17.5±2.1 days. This cycle is composed of diestrus, when the female is sexually inactive and the vagina is closed by an epithelial membrane, followed by the proestrus and estrus phases [32]. In the present study, neonatally thymectomized female guinea pigs displayed no significant difference in the mean length of the estrous cycle (17±0.5 days) when compared to the sham operated animals (16±1.5 days). However, significant differences were observed in the levels of gonadotropins and 17β-estradiol during the estrous cycle between thymectomized and sham-operated female guinea pigs, indicating that neonatal thymectomy primarily affects, components of the neuroendocrine system. The results of this study also confirmed that thymectomy during the neonatal period contributes to estrous cycle dysfunction. In addition, exogenous 17β-estradiol administration has been found to result in regression of the thymus gland and apoptosis in the thymus, in both male and female lizards as well as in mammals, suggesting an inverse relationship between steroid hormone levels and thymic development [45]. Rebar et al. [43,46] reported that reproductive failure in athymic mice is due to poor or absent prepubertal gonadotropin priming. Thymosin β4 can stimulate LHRH secretion from the hypothalamus. Decreased LH concentration is generally considered a good indication of developmental reproductive failure [47]. According to Eppig [48], the culture of ovaries in 8-day-old mice in the absence of gonadotropins resulted in the retardation of follicular growth and the loss of follicular organization, which could be restored by the addition of FSH to the culture media. This implies that thymectomy during the neonatal period can affect the neuroendocrine control of the reproductive axis and lead to alteration of the levels of gonadotropins and 17β-estradiol in the estrous cycle.
In conclusion, the results of the present study clearly demonstrate that neonatal thymectomy can transiently impact the estrous cycle of female guinea pigs. From the reduced levels of gonadotropins and estrogen and the delayed onset of puberty in neonatally thymectomized female guinea pigs, it can be concluded that the thymus either, directly or indirectly, is necessary for the proper development and function of the hypothalamic-pituitary-gonadal axis in the early stages of female guinea pig development. The present experiment may therefore be highly useful in establishing the bases for human and animal reproductive diseases.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Acknowledgements
I am indebted to my colleagues, Dr. S. Pratheepa Shivashangari (professor), Dr. Sai Karthick (assistant professor) and Mrs. Nithya (assistant professor), Department of Anatomy, SRM Medical College Hospital and Research Centre, Kattankulathur, Tamilnadu, India.