Effects of dynamic oxygen concentrations on the development of mouse pre- and peri-implantation embryos using a double-channel gas supply incubator system
Article information
Abstract
Objective
We aimed to evaluate the effects of different oxygen conditions (20% [high O2], 5% [low O2] and 5% decreased to 2% [dynamic O2]) on mouse pre- and peri-implantation development using a novel double-channel gas supply (DCGS) incubator (CNC Biotech Inc.) to alter the oxygen concentration during in vitro culture.
Methods
The high-O2 and low-O2 groups were cultured from the one-cell to the blastocyst stage under 20% and 5% oxygen concentrations, respectively. In the dynamic-O2 group, mouse embryos were cultured from the one-cell to the morula stage under 5% O2 for 3 days, followed by culture under 2% O2 to the blastocyst stage. To evaluate peri-implantation development, the blastocysts from the three groups were individually transferred to a fibronectin-coated dish and cultured to the outgrowth stage in droplets.
Results
The blastocyst formation rate was significantly higher in the low-O2 and dynamic-O2 groups than in the high-O2 group. The total cell number was significantly higher in the dynamic-O2 group than in the low-O2 and high-O2 groups. Additionally, the apoptotic index was significantly lower in the low-O2 and dynamic-O2 groups than in the high-O2 group. The trophoblast outgrowth rate and spread area were significantly higher in the low-O2 and dynamic-O2 groups than in the high-O2 group.
Conclusion
Our results showed that a dynamic oxygen concentration (decreasing from 5% to 2%) had beneficial effects on mouse pre- and peri-implantation development. Optimized, dynamic changing of oxygen concentrations using the novel DCGS incubator could improve the developmental competence of in vitro cultured embryos in a human in vitro fertilization and embryo transfer program.
Introduction
Oxygen is an essential physiological component for regulating embryonic development in the environments of the oviduct and the uterus. Oxygen concentration impacts the rate of embryonic development and the quality of in vitro culture. The majority of modern in vitro fertilization (IVF) labs have accepted a standard of either 5% or 20% oxygen. In the early years of IVF, the embryo was cultured at atmospheric oxygen concentrations (20%); this was proven effective by the birth of millions of children conceived using this method [1,2]. However, many studies have reported that the detrimental effects of oxidative stress during embryonic development at atmospheric oxygen concentrations (20%) were related to an increase in reactive oxygen species (ROS) [3-5]. ROS have been implicated in the damaged development of mammalian embryos in in vitro culture [6]. Exposure to these species causes DNA fragmentation and irreversible doublestrand breaks, which may be especially frequent in the early embryonic stage of development due to active DNA replication during this time. Cellular damage caused by ROS induces uneven embryo cleavage, delayed cleavage, and developmental arrest of the embryo [7]. In addition, hydrogen peroxide, an example of an ROS, is the main mediator of apoptosis and cytoplasmic fragmentation in blastocysts [8].
The physiological oxygen concentration in the mammalian oviduct is around 5% [9-11]. As early as the early 1970s, Steptoe et al. [12] reported that human embryonic development occurred successfully and even improved when using an oxygen concentration of 5%. Other studies have also demonstrated beneficial effects of a 5% oxygen concentration on mammalian embryonic development [13-16]. Furthermore, in mice, an increased blastocyst cell number and improved fetal development have been reported when embryos were cultured in an oxygen concentration of 5% [17-21]. Although most reports have shown that using hypoxic conditions improves embryonic development, the optimal oxygen concentration for developmental stage-specific preimplantation embryos during in vitro culture has not been fully established.
Recently, new suggestions have emerged regarding whether a further reduction of oxygen concentration on day 3 after fertilization represents a physiological condition that is more similar to in vivo conditions during development [11]. These new approaches were based on the premise that the oxygen concentration is actually lower in the uterus than in the oviduct. As the embryo reaches the uterus, it experiences a decrease in oxygen concentration, measured at around 2%, whereas the oxygen concentration in the oviduct is around 5%–7%, at least in nonhuman species [22,23]. Thompson et al. [24] reported that bovine embryonic development was improved by reducing the oxygen concentration from 7% to 2% at the post-compaction stage. Such an oxygen gradient is similar to that in the female reproductive tract, which has a relatively high oxygen concentration in the oviducts (5%–8%) and a very low oxygen concentration in the uterus (approximately 2%). Researchers have attempted to mimic the in vivo environment using an in vitro culture system to study preimplantation embryonic development; however, more research is needed to confirm the hypothesis that we previously described.
In the present study, we hypothesized that sequential exposure to a 5% oxygen concentration from day 1 to day 3 (the pre-compaction stage), and then to a 2% oxygen concentration from day 3 to day 5 (the post-compaction stage), may improve developmental competence compared with continuous exposure to either a 5% or a 20% oxygen concentration. This study was performed to evaluate the effects of dynamic conditions using a novel double-channel gas supply (DCGS) incubator (CNC Biotech Inc., Suwon, Korea) on mouse preand peri-implantation development.
Methods
1. Animals and hormonal stimulation
This study was approved by the Eulji University Institutional Animal Care and Use Committee (No. EUIACUC 17-14). The protocol for superovulated mice was described by Park et al. [25]. Briefly, 6- to 9-week-old female BDF mice were superovulated with intraperitoneal injections of 5 IU of pregnant mare’s serum gonadotropin (Prospec, Rehovot, Israel), and 48 hours later, the mice were injected with 5 IU of human chorionic gonadotropin (Prospec). The superovulated mice were then individually mated with a fertile male BDF mouse.
2. Embryo collection and in vitro culture condition
Once 19 hours had passed after mating, female mice with a confirmed vaginal plug were sacrificed by cervical dislocation, and cumulus-enclosed one-cell embryos (zygotes) were retrieved from the oviductal ampullae. Then, the zygotes were denuded by incubation for 1 minute with 0.1% hyaluronidase (Sigma-Aldrich, St. Louis, MO, USA) in phosphate-buffered saline. The zygotes were pooled and washed three times in Continuous Single Culture-NX (CSCM-NX; FUJIFILM Irvine Scientific, Santa Ana, CA, USA) with 10% human serum albumin (HSA; Irvine). The five healthy zygotes were cultured in 10 μL of CSCM medium with 10% HSA covered with mineral oil for 4 days under three different oxygen conditions.
We used a newly developed incubator with the DCGS system (Figure 1). This incubator could set the conversion timing of different concentrations of O2 gas from 5% to 2%. It is a unique characteristic of the DCGS incubator system. In the dynamic-O2 group, zygotes were cultured from the one-cell to the morula stage under 5% O2 for 3 days, followed by culture under 2% O2 concentration to the blastocyst stage. Embryos in the high-O2 and low-O2 groups were cultured from the one-cell to the blastocyst stage under 20% and 5% oxygen concentrations, respectively. The cleavage rate from the zygote to the two-cell stage and the development rate to the blastocyst stage were determined 24 hours and 96 hours after zygote collection, respectively.
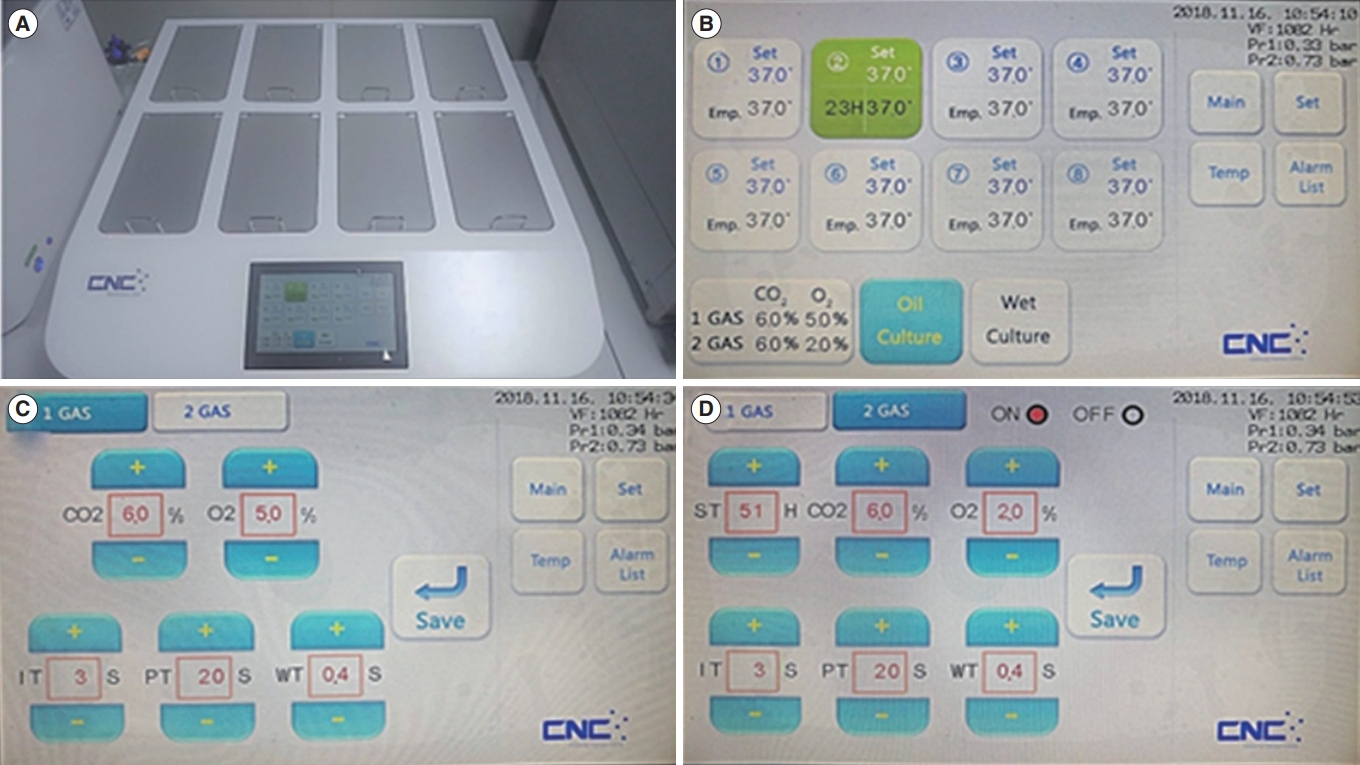
Newly developed incubator with a double-channel gas supply (DCGS) system. (A) Image of a DCGS incubator with eight chambers and a touch-panel screen. (B) Main screen of the DCGS incubator that displays the temperature, incubation time, and gas concentration of each chamber. (C) Screen for controlling the first gas supply of each chamber. (D) Screen for controlling the second gas supply of each chamber.
3. Detection of apoptosis and calculation of the apoptotic index
Apoptotic cells were detected using a fluorescein isothiocyanateconjugated in situ cell death detection kit (terminal deoxynucleotidyl transferase dUTP nick end labeling [TUNEL]; Promega, Durham, NC, USA). The blastocysts were fixed with 4% paraformaldehyde. The apoptotic cells were stained using a TUNEL kit according to the manufacturer’s instructions, and the nuclei were stained in a solution of 10 mg/mL bisbenzimide (Hoechst 33342, Sigma) for 10 minutes prior to observation with a fluorescence microscope (AX-70; Olympus, Tokyo, Japan). Additionally, the number of cells with TUNEL-positive nuclei was determined using an image capturing system (IMT i-Solution, British Columbia, Canada). The apoptotic index was calculated as the percentage of TUNEL-positive nuclei divided by the total number of nuclei in a single blastocyst.
4. Outgrowth assay for evaluation of peri-implantation development
The blastocysts were transferred to a fibronectin-coated dish and outgrown to examine the effects of different oxygen concentrations on peri-implantation embryonic development. The morphological changes during peri-implantation development were observed every 24 hours, and the captured images were analyzed. The area of trophoblastic outgrowth was measured after 72 hours using ImageJ software (National Institutes of Health, Bethesda, MD, USA) as described previously [26].
5. Statistical analysis
All experiments were performed at least in triplicate. All comparisons between groups were determined by one-way analysis of variance. Tukey honest significant difference post hoc test was used for all comparisons between groups. A p-value of less than 0.05 was considered to indicate statistical significance.
Results
1. Effects of different oxygen concentrations on preimplantation embryonic development
Mouse embryonic development rates were assessed following culture for 24 hours (constituting development to the two-cell stage) and 96 hours (constituting development to the blastocyst stage), and are presented in Table 1. No differences were found in the twocell development rate between groups. However, the blastulation rate was significantly higher in the low-O2 (75.7%±2.2%) and the dynamic-O2 (75.6%±4.6%) groups than in the high-O2 (54.0%±2.3%) group (p<0.05).
2. Effects of oxygen concentration on embryo quality
The numbers of total cells and apoptotic nuclei in the blastocysts from each group were determined using Hoechst and TUNEL staining (Figure 2). The mean cell number of the blastocysts was significantly higher in the dynamic-O2 group (128.9±3.3) than in the high-O2 (87.5±2.5) and low-O2 (115.4±3.6) groups. In contrast, the apoptotic index was significantly lower in the dynamic-O2 (4.15%±0.35%) and low-O2 (4.47%±0.49%) groups than in the high-O2 (8.67%±0.82%) group (p<0.05), as shown in Table 2.
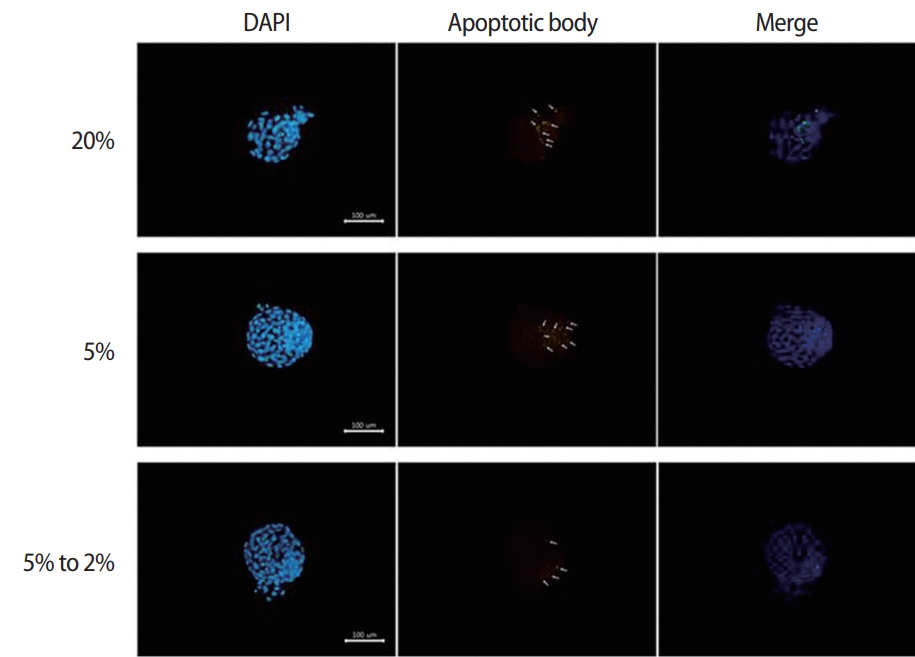
Assessment of apoptotic cells in blastocysts using terminal deoxynucleotidyl transferase dUTP nick end labeling assay. Fluorescence analysis for the number of total and apoptotic cells of the blastocyst. The fragmented nuclei (denoted by green fluorescence) were apoptotic (denoted by white arrows) and DNA was counterstained with the Hoechst stain (denoted by blue). Scale bar, 100 μm. DAPI, 4’,6-diamidino2-phenylindole.
3. Effects of different oxygen concentrations on periimplantation embryonic development
The developmental competence of peri-implantation embryos cultured under different oxygen concentrations was assessed using an in vitro outgrowth assay (Figure 3). After culture of blastocysts for 3 days, the outgrowth rate of the low-O2 (58.3%±2.3%) and dynamic-O2 (71.3%±6.5%) groups was significantly higher than that of the high-O2 (38.4%±0.5%) group. Correspondingly, the trophoblastic spread area in the dynamic-O2 (1.11±0.07 mm2) and low-O2 (1.05±1.11 mm2) groups was significantly wider than that in the high-O2 (0.70±0.05 mm2) group (p<0.05), as shown in Table 3.
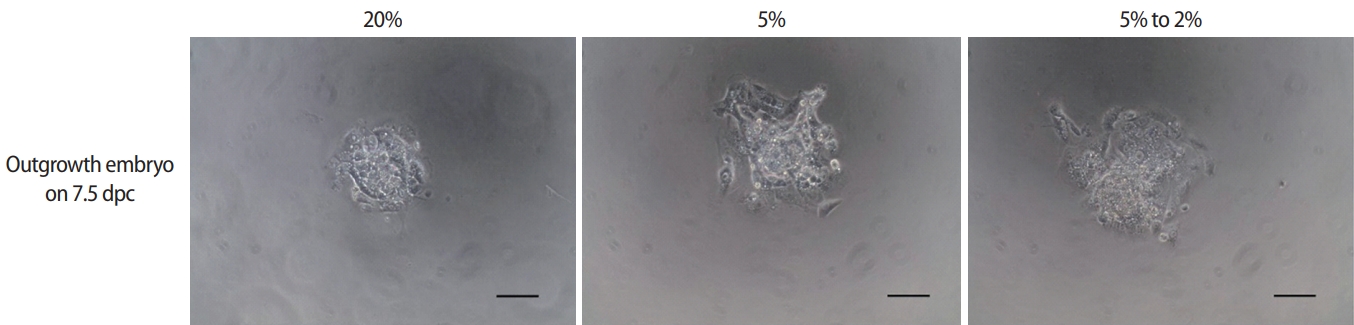
Effects of different oxygen concentrations on outgrowth embryos after 3-day culture of blastocysts from different groups. Morphologic assessment of outgrowth embryos under high (20%), low (5%), and dynamic (5% decreasing to 2%) oxygen concentrations. Scale bar, 100 μm.
Discussion
This study demonstrated that the in vitro culture of mouse embryos under 5% to 2% oxygen concentration (the dynamic-O2 group) with a DCGS incubator system improved development of mouse pre- and peri-implantation embryos compared with low (5%) and high (20%) oxygen concentrations. We consistently found adverse effects of an atmospheric oxygen concentration (20%) on mouse embryonic development during in vitro culture.
Oxygen concentration affects the rate of in vitro embryonic development and quality. The majority of modern IVF clinics have used 5% or 20% oxygen concentrations when culturing embryos under in vitro incubation. However, controversy still exists regarding whether a further reduction of oxygen concentration on day 3 after fertilization (the post-compaction stage) represents a physiological state that is more similar to in vivo oxygen conditions. This approach is based on the assumption that the physiological oxygen concentration is actually lower in the uterus (at approximately 2%) than in the oviduct (at 5%–7%) [9-11,22,23].
In a previous report, bovine embryos were cultured at various oxygen concentrations (0%, 1%, 2%, 4%, and 7%), and these embryos displayed the highest blastocyst development rate under the 2% oxygen concentration condition [24]. Furthermore, Kaser et al. [27] examined the blastocyst development potential of normal and abnormal embryos after culture under 2% or 5% oxygen concentrations from the post-compaction stage. Embryos cultured under a 2% oxygen concentration displayed a higher blastulation rate and a higher utilization rate. However, in our results, the blastulation rate in the 5% to 2% oxygen concentration group was not significantly different from that in the 5% oxygen concentration group, although a significantly higher development rate was observed than in the 20% oxygen group. Similarly, Feil et al. [28] examined mouse embryo culture at a 7% oxygen concentration from the zygote stage embryo to the morula stage, and surviving morulae were then randomly cultured to the blastocyst stage at 2%, 7%, or 20% oxygen. In the results of that study, no differences were found in the blastulation rate among these groups. In studies of human embryos, there were no significant differences in the development rate to the blastocyst stage or in the number of high-quality blastocysts when embryos were cryopreserved on day 3 and cultured to the blastocyst stage at 2%, 5%, or 20% oxygen [29]. This study applied dynamic oxygen conditions, decreasing from 5% to 2%; these concentrations were determined through careful review of many previous studies and a brief preliminary experiment (data not shown).
Oxygen concentrations during the in vitro culture of embryos, from 20% to lower levels, were related to embryonic development and embryo quality. The blastulation rate and the total cell number of blastocysts were related to subsequent fetal viability [30]. Many studies have shown that low oxygen concentrations were beneficial to embryonic development compared to higher oxygen concentrations [13,17,19,24,31-36]. Those previous studies used a single oxygen concentration, as the automatic oxygen-changing incubator system used in our study was not available. A recent study conducted by De Munck et al. [37] compared the effects of direct or gradual exposure to 2% or 5% oxygen on the in vitro culture of human post-compaction embryos. No significant difference was found in blastulation and the good-quality embryo rate between the 2% and 5% oxygen concentrations.
Our study, unlike most previous studies, developed and applied a novel DCGS incubator to automatically change the oxygen concentration. This experiment incorporated sequential exposure to a 5% oxygen concentration from day 1 to day 3 (the pre-compaction stage), and then to a 2% oxygen concentration from day 3 to day 5 (the post-compaction stage), which was determined in consideration of the physiological oxygen conditions of the female reproductive tract. This DCGS incubator system may be advantageous in that it can automatically change the oxygen concentration from 5% to 2% without any interference or adaptation time. In this study, the total cell number of blastocysts was significantly higher in the dynamic-O2 (5% to 2%) group compared with the low-O2 (5%) and high-O2 (20%) groups. The apoptotic index of the dynamic-O2 (5% to 2%) group was also the lowest of the three groups. In contrast, Yang et al. [29] reported that there was no significant difference between the total cell number of cultured blastocysts in 2% and 5% oxygen after the post-compaction stage compared to the 20% oxygen condition. The difference between that study and our study is the oxygen concentration of the pre-compaction stage from day 1 to day 3 (20% vs. 5%, respectively). It can be assumed that 20% oxygen induced irreversible damage to the blastocysts via abundant ROS products.
Blastocyst outgrowth has been used as an in vitro model for the implantation of developing embryos in utero. It could be applied for various studies of trophoblasts physiology and implantation competence in peri-implantation embryos [26,38,39]. Aspects of the in vitro trophoblastic outgrowth model, such as activation and spread, are related to proliferation and invasion in in vivo implantation processes [40-43]. In addition, morphokinetics and metabolism, as evidenced by the trophoblastic outgrowth assay, are correlated with viability and implantation potential [44,45]. In this study, the outgrowth rate after 3 days of culture of blastocysts in the dynamic-O2 (5% to 2%) and low-O2 (5%) groups was superior to that in the high-O2 (20%) group. Additionally, the mean trophoblastic spread area of the dynamic- and low-O2 groups was significantly wider than that of the high-O2 group. These results show that preimplantation embryos cultured under dynamic O2 (5% to 2%) concentrations could have a greater viability and implantation potential. There is a limitation of this study to not fully excluding exposure to 20% oxygen in the air during sampling and handling of mouse embryos. There is a limitation of this study to not fully excluding exposure to 20% oxygen in the air during sampling and handling of mouse embryos. Nonetheless, in the in vitro culture of preimplantation embryos, optimized oxygen concentrations similar to those in vivo should be considered as a necessary factor for improving culture conditions.
In conclusion, the results of this study demonstrate the beneficial effects of dynamic oxygen concentrations, shifting from 5% to 2% on day 3. This may be related to the physiological conditions experienced by preimplantation embryos in the uterus. We recommend that future studies include more samples, and we posit that embryo transfer in vivo is also required to fully investigate the implantation potential of in vitro cultured embryos. Additionally, more research should explore the application of other oxygen conditions during embryo culture. We suggest that the optimized and dynamic changing of oxygen concentrations with the novel DCGS incubator could improve the developmental competence of in vitro cultured embryos in human IVF/embryo transfer programs.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Author contributions
Conceptualization: JHJ, KWC. Data curation: SCL, JL, HCS. Method ology: SCL, JL, HCS, JHJ. Project administration: JHJ, KWC. Writing - original draft: SCL, JHJ. Writing - review & editing: JL, HCS, KWC.



