Understanding the molecular mechanisms of bisphenol A action in spermatozoa
Article information
Abstract
Bisphenol A (BPA) is an endocrine-disrupting chemical that is capable of interfering with the normal function of the endocrine system in the body. Exposure to this chemical from BPA-containing materials and the environment is associated with deleterious health effects, including male reproductive abnormalities. A search of the literature demonstrated that BPA, as a toxicant, directly affects the cellular oxidative stress response machinery. Because of its hormone-like properties, it can also bind with specific receptors in target cells. Therefore, the tissue-specific effects of BPA mostly depend on its endocrine-disrupting capabilities and the expression of those particular receptors in target cells. Although studies have shown the possible mechanisms of BPA action in various cell types, a clear consensus has yet to be established. In this review, we summarize the mechanisms of BPA action in spermatozoa by compiling existing information in the literature.
Introduction
Bisphenol A (BPA) is a major environmental pollutant widely used to manufacture numerous consumer products, including food packaging materials, industrial supplies, dental sealant, and thermal paper receipts. Exposure to this chemical through the oral, respiratory, and dermal routes is ubiquitous in both humans and animals [1,2]. Originally, BPA was used as a growth promoter for cattle and poultry [3], but it was later proven that this chemical interferes with the regular hormonal functions of the body, subsequently predisposing individuals to deleterious health effects [4-6]. As summarized by Rochester [7], exposure to BPA is associated with cardiovascular diseases, abnormalities of brain development, obesity, thyroid dysfunction, diabetes, breast cancer, and infertility.
As a male reproductive toxicant, exposure to this chemical is associated with abnormalities of testicular cell function and spermatogenesis, a decrease in sperm production and formation of morphological abnormal spermatozoa, defects of accessory sex gland function, and subfertility/infertility [7-9]. BPA, at the dose currently considered acceptable, is also capable of interfering with reproductive and developmental defects in laboratory animals, aquatic species, and wildlife [1,10,11]. However, the current understanding of BPA action in cells is mostly uncertain. In this review, we summarize the mechanism of BPA action in spermatozoa by compiling existing information in the literature. In particular, we discuss how BPA affects spermatozoa, because the spermatozoon is an excellent model for understanding the effects of BPA on male fertility and reproduction.
Molecular mechanisms of BPA action in spermatozoa: in vitro
In vitro experimental models have greatly advanced our understanding of how to screen for chemical toxicity from the public health perspective. In addition, in vitro models are rapid, economical, and require less labor to make preliminary assumptions about a toxic chemical. In fact, our current understanding of the action of BPA is mostly derived from in vitro experimental findings. In this section, we review how BPA affects sperm function and fertility following direct in vitro exposure.
1. BPA-induced compromise of cellular stress response mechanisms
Spermatozoa are highly susceptible to oxidative stress because they lose the majority of their cytoplasm in the final stage of spermatogenesis [12]. Oxidative stress facilitates cellular damage by increasing levels of oxygen and oxygen-derived oxidants, commonly known as reactive oxygen species (ROS). Although ROS are important for normal sperm function at low levels, excessive levels of ROS have detrimental effects on motility, capacitation, and the acrosome reaction of spermatozoa [13,14]. Furthermore, high levels of ROS can damage lipids, proteins, and DNA in spermatozoa [15]. Recently, we showed that exposure of mouse spermatozoa to 100-μM BPA potentially arrested the oxidative stress response mechanism, as measured by elevated ROS [5,6]. In addition, we also reported that a BPA-induced increase in ROS levels in spermatozoa was associated with a premature acrosome reaction, loss of sperm motility, viability, ionic imbalance, and alterations of the sperm proteome (Figure 1) [5,6]. Increased ROS and lipid peroxidation have also been reported by other recent studies following in vitro exposure to BPA in spermatozoa and other cells [16]. Thus BPA, as a toxic chemical, compromises the oxidative stress response machinery of spermatozoa, thereby affecting their ability for fertilization.
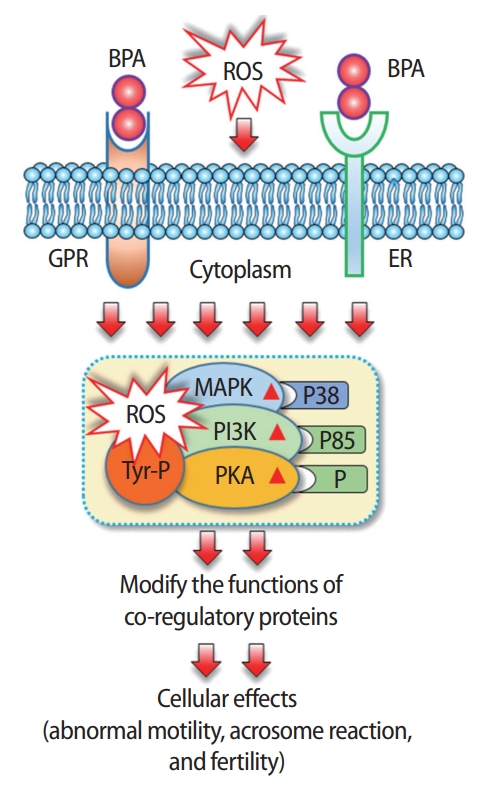
Changes in spermatozoa following exposure to bisphenol A (BPA) in vitro. BPA may bind with either G protein-coupled receptors (GPR30) or estrogen receptors (ERs) located in the plasma membrane. BPA-mediated activation of these non-genomic receptors or increased stress caused by elevated reactive oxygen species (ROS) in spermatozoa rapidly phosphorylates (P) mitogen-activated protein kinase (MAPK), phosphatidylinositol 3-kinase (PI3K), and protein kinase A (PKA). In addition, it causes phosphorylation of sperm proteins in tyrosine residues (Tyr-P). All these conditions are responsible for the modification of coregulatory function and subsequently predispose individuals to abnormal cellular outcomes.
2. Receptor-dependent mechanisms of BPA action
Circulating hormones come into contact with all cells in the body; however, they only affect the target cells that contain specific receptors for the hormone. Since BPA possesses hormone-like properties, its action in target cells is mostly related to the availability of particular receptors in cells. A comprehensive search of the literature demonstrated that BPA acts as an estrogen receptor (ER) modulator [6], stimulates estrogen-related receptor gamma [17] and growth factor receptors [18], and exerts anti-androgenic [19] and anti-thyroid activity [20]. Recently, Naz and Sellamuthu [21] reported that mature mammalian spermatozoa express ERα, ERβ, growth factor receptors, and androgen receptors, meaning that the effects of BPA on spermatozoa could also be regulated via receptor-dependent signaling. To date, several mechanisms of BPA action have been investigated, but particular attention has been directed towards its ability to bind with ER in various cell types, including spermatozoa. A dynamic simulation interaction of BPA with a typical ERα is shown in Figure 2. ERα contains a missing residue at 3UU7 that could bind with BPA, and doing so keeps the receptor in its active conformation.
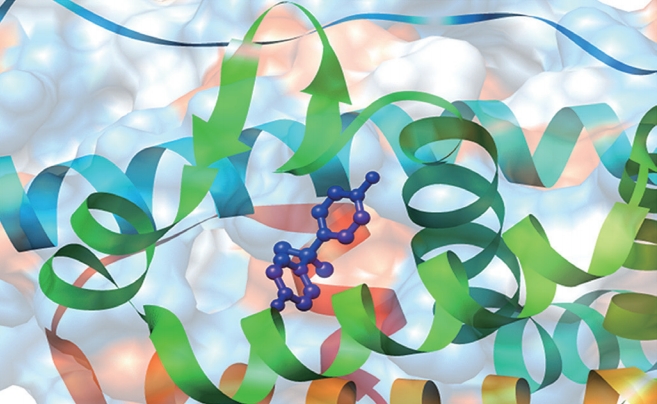
Interaction mechanism of bisphenol A (BPA) with estrogen receptors (ERs). A dynamic simulation demonstrating the interaction mechanism between BPA and a typical ERα. The atom coordinates of the complexes of ERα-BPA (PDB code: 3UU7, 2.2 Å) were obtained from the RCSB Protein Data Bank. As there were missing residues in 3UU7, Chimera 1.13 was used to add these missing residues in the loop regions. The added loops were refined through the loop refinement protocol. This structural analysis suggested that BPA could interact with the receptors on spermatozoa by mimicking the action of a natural hormone and keeping the ERs in an active conformation.
As a ER modulator, BPA works via genomic and non-genomic pathways (Figure 3). In the genomic pathway, it binds with the ERs located in the cytoplasm (cERs) or directly binds with the ERs located in the nucleus (nERs). The binding modifies overall receptor signaling, affects nuclear chromatin function, and regulates the transcription/translation of proteins/genes, thereby affecting cell proliferation, differentiation, and survival [6,22]. In non-genomic signaling, BPA interacts with G protein-coupled receptors (GPR30) and membranebound ERs (mERs). Activation of these receptors triggers rapid estrogenic signaling via activation of cellular kinase systems such as protein kinase A (PKA), mitogen-activated protein kinase (MAPK), phosphatidylinositol 3-kinase (PI3K), and changes in levels of cyclic adenosine monophosphate, protein kinase C, and intracellular calcium, as demonstrated in the pancreatic islets [18], and human ovarian cancer cells (Figure 3) [23,24]. However, genomic ERs (e.g., cERs and nERs) are absent in mature spermatozoa; therefore, BPA mostly affects spermatozoa through non-genomic pathways by interacting with GPR30 and mERs [6]. Recently, we reported that exposure of mouse spermatozoa to >1 μM BPA led to the rapid phosphorylation of p38, p85, PKA, and tyrosine [5,6]. Phosphorylation of p38 and p85 represents the activation of the MAPK and PI3K kinase system in cells, respectively. In support of our observation, Wan et al. [25] also reported an increase in PKA and tyrosine phosphorylation in spermatozoa following in vitro exposure to BPA (Figure 1).
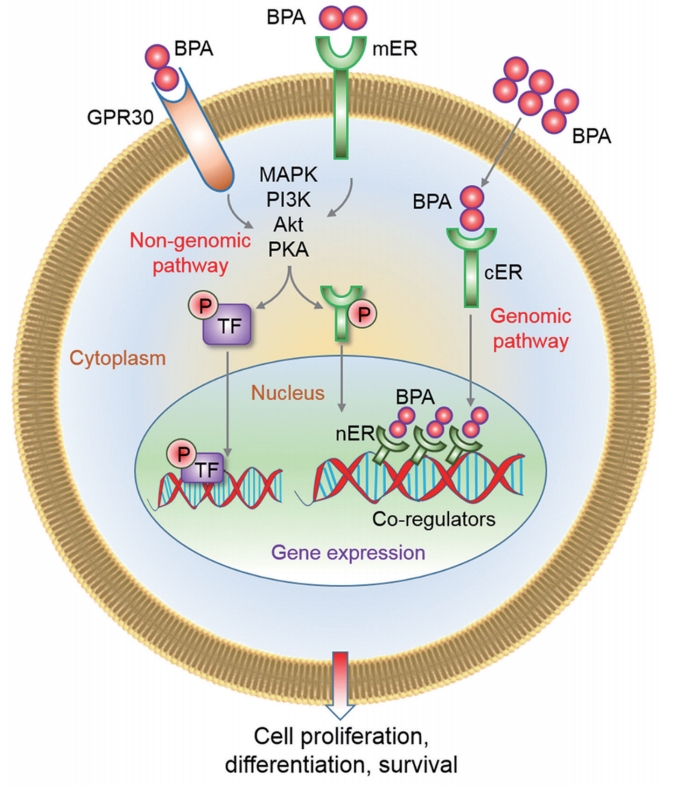
Mechanisms of bisphenol A (BPA) action through the modification of estrogen receptors (ERs). Both genomic and non-genomic pathways have been highlighted in this figure. A detailed description of each pathway is given in the main text of the manuscript. GPR30, G protein-coupled receptors; mER, membrane-bound ER; MAPK, mitogen-activated protein kinase; PI3K, phosphatidylinositol 3-kinase; PKA, protein kinase A; cER, cytoplasmic ER; P, phosphorylation; TF, transcription factor; nER, nuclear ER.
In both genomic and non-genomic signaling, modified ERs in cells alter the activities of coregulatory proteins/genes via transcriptional and translational modifications [22]. However, mammalian spermatozoa are silent in both transcription and translation [2,26-28]. Therefore, it is difficult to explain BPA-mediated estrogenic effects in spermatozoa. Notably, post-translational modifications (such as protein phosphorylation, glycosylation, and so on) are very common in spermatozoa and play a major role in modifying the sperm proteome, which is responsible for the functional maturation of spermatozoa before fertilization [1,29]. Therefore, together with other researchers in this field, we suggest that the modification of coregulatory proteins in spermatozoa occurs mostly because of posttranslational modification of these proteins (Figure 1).
As an androgen receptor antagonist, BPA was found to inhibit the amino- and carboxyl-terminal regions of androgen receptors in monkey kidney cells [30]. Inhibition of androgen receptors through this mechanism subsequently activates interaction of these receptors with the silencing mediator for thyroid hormone receptors and the nuclear receptor co-repressor, subsequently suppressing cell proliferation [30]. However, similar mechanisms have not yet been studied in spermatozoa.
Mechanisms of BPA action in spermatozoa following in vivo exposure
The effects of BPA on spermatozoa in vivo are mostly exerted via the alteration of the hormonal functions in the body that regulate testicular spermatogenesis, the process of mammalian sperm production. Testicular cells (such as Sertoli and Leydig cells) play a critical role in the development of germ cells/spermatozoa. Although the factors regulate spermatogenesis are not completely understood, complex hormonal messaging through endocrine, paracrine, and autocrine signaling is required for the production of healthy spermatozoa [31,32]. BPA may affect spermatozoa through the following pathways, by manipulating the testicular environment or affecting testicular cell function.
1. Manipulation of reproductive hormone functions
Mammalian spermatogenesis is coordinated by the hypothalamicpituitary-testicular axis and the activity of the thyroid hormones [20]. Endocrine-disrupting chemicals such as BPA can easily disrupt this axis, thereby affecting/halting spermatogenesis. Increased serum levels of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) and decreased levels of testosterone were reported in men in Guizhou Province, China following environmental exposure to BPA [33]. The anterior pituitary releases FSH, which increases the production of androgen-binding protein in Sertoli cells to enhance testicular growth. Simultaneously, LH from the same origins acts on testicular Leydig cells to stimulate testosterone production, which also plays a major role in spermatogenesis [33]. Meanwhile, reduced thyroid hormone levels were reported in newborn babies following prenatal BPA exposure [34]. Although an earlier investigation suggested that thyroid hormone plays a negligible role in male fertility, it is now being recognized that it exerts a significant effect on sperm production [35]. Similar effects of BPA on gonadotropic and thyroid hormones have been reported in many in vivo animal studies (reviewed in [36]) by which it could affect the production and function of spermatozoa.
2. Effects of BPA on spermatogenesis: receptor-dependent mechanisms
BPA may also affect the spermatogenesis process via antiestrogenic and androgenic pathways. It has been reported that exposure of male Wistar rats to BPA for 9 days significantly increased the proportion of stage VII seminiferous epithelium and decreased the amount of stage VIII seminiferous epithelium in the testis [37]. Apoptosis of testicular germ cells at stages VII and VIII was also shown in another study following exposure to 17-β estradiol in male rats [38]. The transformation of spherical spermatids into elongated spermatozoa is known as spermiogenesis, which occurs in stage VII and VIII seminiferous epithelium. Therefore, alteration of these stages, including apoptosis, is directly linked to the reduction of sperm concentration [38]. BPA-mediated adverse effects on spermatogenesis also have been reported in male goldfish, along with alterations of total sperm number, volume, and motility [39,40].
Recently, we have shown that gestational exposure to BPA for 7 days decreased the concentration and motility of F1 spermatozoa during adulthood [1]. This is because of a decrease in the number of stage VIII seminiferous epithelial cells in the testis and a decline in intracellular ATP levels in F1 male spermatozoa. Testicular development starts at the early stage of fetal development. BPA is capable of passing through the placenta to a developing embryo; therefore, a developing fetus has a high risk of BPA exposure from endogenous sources. As such, BPA can bind with ERs located in the testis and causes harmful effects on germ cell differentiation, sperm production, and levels of male reproductive hormones during adulthood [41]. In fact, early testicular development is highly sensitive to estrogens and to estrogenic compounds such as BPA. Delbes et al. [42] reported that mouse germ cells expressed ERβ from 14 to 26 days post-conception, and that inactivation of ERβ led to a 50% increase in the germ cell population. In another study, Jefferson et al. [43] reported the expression of ERα mRNA in the testicular interstitial cells (Leydig) cells at 26 days after birth, and its function was found to be related to testosterone hormone signaling (Figure 4). Therefore, BPA may affect the testicular cell population and spermatogenesis through a receptor-dependent signaling pathway. However, further studies are required to prove this hypothesis conclusively.
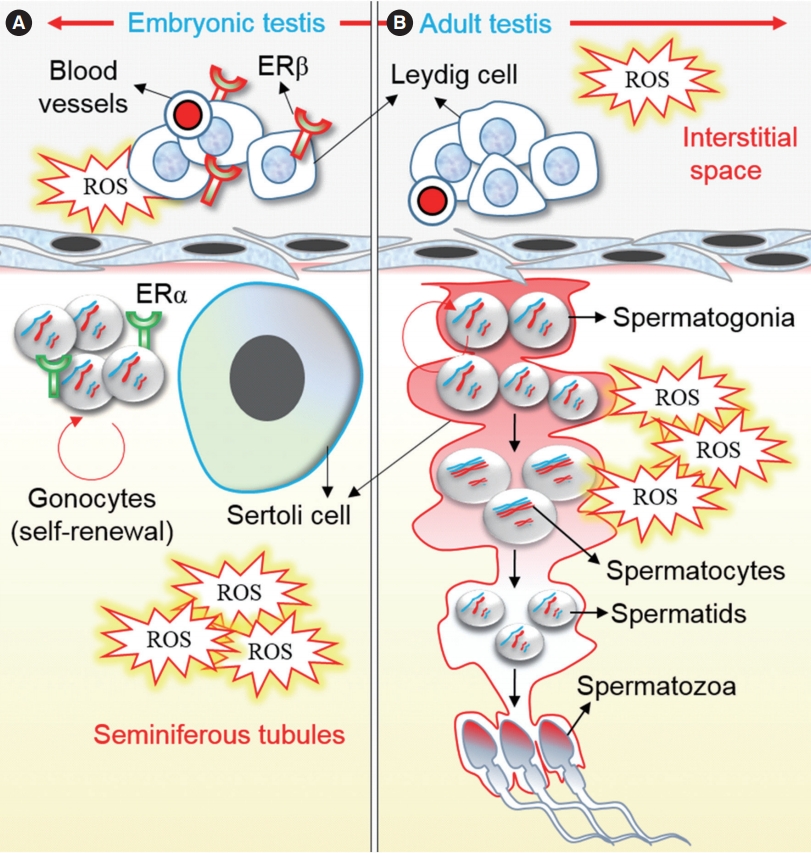
An outline of the distribution of estrogen receptors (ERα and ERβ) in the developing testis (A) and the possible mechanism of bisphenol A (BPA) action in testicular and germ cells in the developing (A) and adult testis (B). Reactive oxygen species (ROS) have a major detrimental effect on testicular cells and on the growth and differentiation of germ cells, as mediated by developmental exposure to BPA. A detailed description of each step is provided in the main text.
3. BPA-mediated oxidative stress in testicular cells
Physiologically, the oxygen levels in the testis are lower than in other cells/tissues because of functional weakness of the testicular artery. To maintain very high levels of cell division for germ cell production/differentiation, testicular cells are in severe competition for oxygen [44]. Therefore, testicular cells and the process of spermatogenesis are extremely prone to oxidative stress, which may lead to abnormal sperm production and the generation of morphologically abnormal spermatozoa. A recent study conducted by Kaur et al. [45] demonstrated that BPA exposure for 8 weeks in male mice was responsible for a dramatic increase in the testicular ROS and lipid peroxidation, with associated loss of sperm concentration and motility. Consistently, male rats exposed to BPA (10 mg/kg body weight) for 14 days were found to have increased testicular oxidative stress, as detected by elevated antioxidant enzymes such as glutathione reductase, glutathione peroxidase, superoxide dismutase, and catalase in the testis [46]. When BPA-exposed rats were fed α-lipoic acid (an antioxidant) [47], testicular mitochondrial toxicity/oxidative stress conditions were significantly overcome [46]. In a recent study, we showed that exposure of pregnant mice to BPA for 7 days increased oxidative stress in spermatozoa even in F1 male mice during adulthood [1]. Increased oxidative stress directly affects DNA, lipids, and antioxidant enzymes in germ cells, subsequently causing apoptosis and leading to a decline in semen parameters associated with male infertility [48]. These findings have led researchers to believe that apart from its major endocrine-disrupting properties, BPA also perturbs the pro-oxidant and anti-oxidant balance in testis cells, thereby affecting spermatozoa.
4. BPA-induced modification of sperm epigenetics
Growing evidence supports the impact of lifestyle and environmental factors, not only on the health of an exposed individual, but also influencing the phenotypes of further generations [49-51]. This takes place via parental germ-line alterations, such as the transmission of paternal information to the offspring by remodeling the epigenetic programming of spermatozoa. Although the basic concept of epigenetics is not a new idea, the field of environmental epigenetics has significantly expanded in the last decade. A search of the literature demonstrated that parental exposure to BPA is capable of modifying sperm epigenetics [50], notably by DNA methylation, histone modifications, and interference of non-coding microRNAs in spermatozoa, thereby leading to transgenerational effects. In a pioneering study, Manikkam et al. [52,53] demonstrated an increased disease incidence in F3-generation male and female rats with associated differential DNA methylation regions in the sperm epigenome following gestational exposure to BPA in F0 mothers. Recently, altered protein expression in the spermatozoa (both capacitated and non-capacitated) of F1 male mice was reported by our group following gestational exposure to BPA [1,2]. However, similar studies have yet to be conducted to elucidate a more detailed understanding of the BPA-mediated perturbation of offspring health mediated via sperm epigenetic transmission.
Conclusions
Exposure to environmental contaminants may be the most prominent factor affecting male fertility/reproduction. As a common environmental contaminant, developmental exposure to BPA is associated with deleterious health effects in both animals and humans, including many aspects of reproduction in exposed individuals, as well as their offspring in subsequent generations. Simultaneously, the effects of BPA are especially severe when the exposure is associated with other risk factors, such as poor diet and concurrent diseases. Leung et al. [54] reported that BPA exposure in pregnant rats fed a high-butterfat diet increased the incidence of mammary tumors in offspring in comparison to rats with a high-butterfat diet only. Moreover, male obesity was found to be associated with a reduction in sperm quality, as manifested by low sperm count and motility [55]. Therefore, BPA-mediated adverse effects on fertility/reproduction in obese individuals might more prominent and could follow different mechanisms of action. Therefore, it is very difficult to hypothesize which mechanism BPA follows to affect spermatozoa or other cells under a particular exposure condition. However, it is true that BPA, at high doses, strongly suppresses oxidative stress machinery in spermatozoa following in vitro exposure. In contrast, in in vivo conditions, BPA may trigger different signaling cascades in spermatozoa, modify the sperm epigenetic machinery, and impact the growth and development of the testicular cell population, including spermatozoa. Thus, more studies should be conducted in the near future to elucidate the exact molecular mechanism of BPA action in spermatozoa and other cells.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Author contributions
Conceptualization, Funding acquisition, Writing - original draft, review & editing: all authors.
