Analysis of vitamin D-binding protein (VDBP) gene polymorphisms in Korean women with and without endometriosis
Article information
Abstract
Objective
Vitamin D-binding protein (VDBP) mediates various biological processes in humans. The goal of this study was to investigate whether VDBP gene polymorphisms could predispose Korean women to endometriosis.
Methods
We prospectively enrolled women with endometriosis (n = 16) and healthy controls (n = 16). Total serum 25-hydroxyl vitamin D (25(OH)D) concentrations were measured using an Elecsys vitamin D total kit. Levels of bioavailable and free 25(OH)D were calculated. Concentrations of VDBP were measured using a vitamin D BP Quantikine ELISA kit. DNA was extracted using a DNeasy blood & tissue kit. Two single-nucleotide polymorphisms (SNPs; rs4588 and rs7041) in GC, the gene that codes for VDBP, were analyzed using a TaqMan SNP genotyping assay kit. The functional variant of VDBP was determined based on the results of the two SNPs.
Results
Gravidity and parity were significantly lower in the endometriosis patients than in the control group, but serum CA-125 levels and the erythrocyte sedimentation rate were significantly higher. Total serum 25(OH)D levels in the endometriosis patients were significantly lower than in the control group. However, serum bioavailable 25(OH)D, free 25(OH)D, and VDBP levels did not differ significantly between the endometriosis and control groups. The genotypes and allele frequencies of GC were similar in both groups.
Conclusion
Korean women with endometriosis had lower total serum 25(OH)D concentrations than controls. Neither serum VDBP concentrations nor polymorphisms in the gene coding for VDBP were associated with endometriosis. Further studies are needed to investigate the pathophysiology and clinical implications of 25(OH)D and VDBP in endometriosis.
Introduction
Endometriosis is a chronic, benign, estrogen-dependent, inflammatory disease affecting approximately 10% of reproductive-age women and 35%–50% of women with pelvic pain and infertility. Endometriosis, which is defined as growth of endometrial tissue outside the uterine cavity, often results in a vast array of gynecological problems, including dyspareunia, dysmenorrhea, pelvic pain, and infertility [1].
The cause of endometriosis is not completely clear. Numerous hypotheses have been proposed to explain the presence of ectopic endometrial tissue and stroma. For example, it has been hypothesized that retrograde menstruation and metaplasia play a role in the formation of ectopic endometrium in endometriosis [2]. However, retrograde menstruation alone is unable to explain all instances of endometriosis. Additional factors such as genetic and immune differences need to be invoked to account for the fact that many women with retrograde menstruation do not have endometriosis [3].
The observation that autoimmune diseases are more common in women with endometriosis supports the possibility that the pathogenesis of endometriosis may involve a defective immune response [4]. Regurgitation of endometrial cells into the peritoneum can trigger an inflammatory response that recruits activated macrophages and leukocytes locally [5,6]. This inflammatory response may cause defective “immune-surveillance,” which prevents elimination of menstrual debris and promotes the implantation and growth of endometrial cells at ectopic sites [7].
Humans acquire vitamin D from exposure to sunlight and dietary intake. Acquired vitamin D is metabolized in the liver, resulting in 25-hydroxyl vitamin D (25(OH)D) [8,9]. Most (85%–90%) of circulating 25(OH)D is tightly bound to vitamin D-binding protein (VDBP), with a smaller amount (10%–15%) loosely bound to albumin. Less than 1% of circulating vitamin D exists in a free unbound form [10]. The fraction not bound to VDBP (free and albumin-bound forms) is considered as bioavailable 25(OH)D.
Vitamin D is a multipotent vitamin that has a hormone-like function, including endocrine functions, regulation of cell replication, and immune modulation. The effects of vitamin D on the immune system and immune-related diseases have become the subject of a large number of studies. In this context, it has been discovered that supplementation with 1,25(OH)2D3 could prevent both the initiation and progression of experimental autoimmune encephalomyelitis and collagen-induced arthritis in experimental models of multiple sclerosis and rheumatic arthritis, respectively [11,12].
In humans, VDBP, originally known as GC-globulin (group-specific component), is encoded by the GC gene [13]. VDBP belongs to the albumin gene family, together with human serum albumin and alphafetoprotein. It is a multifunctional protein found in plasma, ascitic fluid, and cerebrospinal fluid, as well as on the surface of numerous cell types. VDBP is able to bind various forms of vitamin D, including ergocalciferol (vitamin D2), cholecalciferol (vitamin D3), 25-hydroxylated forms (calcifediol), and 1,25(OH)2D3 (calcitriol, the active form). The majority of vitamin D in the blood is bound to VDBP, which transports vitamin D metabolites from the skin, liver, and kidney to various target tissues [14]. VDBP is also known to mediate various biological functions in humans, including immune modulation, osteoclast activation, activation of chemotaxis, and fatty acid transport [15].
VDBP is the precursor molecule of a potent macrophage-activating factor [16] that is highly tumoricidal against various malignancies through its ability to inhibit endothelial angiogenesis [17] and stimulate inflammation-primed phagocytic activity of tumoricidal macrophages [18]. Therefore, researchers in a previous study hypothesized that VDBP might play an important role in colorectal cancer initiation and progression, either alone or in combination with vitamin D [19].
The GC gene, which encodes VDBP, is highly polymorphic. Polymorphic VDBP proteins differ in their affinity for the 1,25(OH)2D metabolite. Two common coding single-nucleotide polymorphisms (SNPs)—rs7041 (Asp416Glu) and rs4588 (Thr420Lys)—are correlated with circulating vitamin D levels. In addition, the combination of the two SNPs, rs7041 and rs4588, produces three variant combinations (Gc1s, Gc1f, and Gc2). These variants provide different binding affinities for vitamin D metabolites. It has been reported that Gc1f has the greatest affinity, followed by Gc1s and Gc2 [20]. A previous study has demonstrated that GC SNPs may affect blood plasma vitamin D levels [21], implying that they could be a risk factor related to the pathogenesis of endometriosis [22].
Endometriosis is a multifactorial disease in which local inflammatory processes appear to play a key role in development and progression [6]. Increasing interest in vitamin D has emerged because of its role as an immune modulator [23]. Although few studies have investigated the correlations of VDBP gene polymorphisms with bioavailable 25(OH)D and free 25(OH)D in patients with endometriosis, Faserl et al. [24] suggested that VDBP gene polymorphisms might be a risk factor for endometriosis.
Based on this observation, we hypothesized that VDBP gene polymorphisms might have relationships with endometriosis, as well as serum vitamin D (total 25(OH)D, bioavailable 25(OH)D, and free 25(OH)D) concentrations. To test this hypothesis, we analyzed serum vitamin D levels and VDBP gene polymorphisms in 16 patients with endometriosis and 16 healthy controls.
Methods
1. Study subjects
This was a prospective study conducted at the Gyeongsang National University Hospital, Jinju, Korea. Whole blood and serum samples were collected from 16 patients with endometriosis and 16 healthy controls between February 2017 and March 2019. All patients with endometriosis had a definitive diagnosis made based on a pathological review of their surgical specimens. Healthy controls were defined as women who visited Gyeongsang National University Hospital for a health checkup without having any symptoms of endometriosis. Healthy controls were enrolled in this study only if they did not have any disease (such as uterine leiomyomas; hypertension; diabetes; multiple sclerosis; autoimmune disorders; or coronary, hepatic, or renal disease as assessed through a questionnaire and laboratory tests) that could affect serum VDBP concentrations. If other underlying diseases were present, patients were excluded from this study. In addition, all potential participants were surveyed to determine whether they had a recent history of taking vitamin D supplements and hormonal pills. Only those who did not have such a history were included in this study. This study was approved by the Institutional Review Board of Gyeongsang National University Hospital (IRB No. GNUH 2017-01-004).
2. Serum total 25(OH)D assay and calculation of bioavailable and free 25(OH)D
Each serum sample was aliquoted into two tubes and stored at −80°C until it was analyzed for VDBP and total 25(OH)D. Concentrations of VDBP were measured using a human vitamin D BP Quantikine ELISA kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s protocol. Measurements of total serum 25(OH)D concentrations were performed using an Elecsys vitamin D total kit with a Cobas e602 module (Roche Diagnostics, Mannheim, Germany). This was an electrochemiluminescent assay with ruthenium-labeled VDBP, biotin-labeled vitamin D, and streptavidin-coated microparticles.
Levels of bioavailable and free 25(OH)D were calculated from the total measured 25(OH)D, VDBP, and serum albumin concentrations using the following equations [25,26].
To calculate the genotype-specific bioavailable 25(OH)D levels, GC genotype-specific VDBP binding affinity (KVDBP1f, 1.12×109 M–1; KVDBP1s, 0.6×109 M–1; KVDBP2, 0.36×109 M–1) was used instead of generic KVDBP [27]. For heterozygous genotypes, the mean affinity of the two homozygotes was used (KVDBP1f/1s, 0.86×109 M–1; KVDBP1f/2, 0.74×109 M–1; KVDBP1s/2, 0.48×109 M–1) [28]. In this study, bioavailable 25(OH)D referred to both genotype-constant and genotype-specific bioavailable 25(OH)D.
3. Genotyping of VDBP
DNA was extracted from all samples using a DNeasy blood & tissue kit (250; Qiagen, Hilden, Germany) following the manufacturer’s instructions. Two VDBP SNPs (rs4588 and rs7041) were analyzed using realtime polymerase chain reaction with a TaqMan SNP genotyping assay kit (Thermo Fisher Scientific, Waltham, MA, USA). The functional VDBP variant was determined based on results of the 2 SNPs (rs7041 and rs4588). If there was no nucleotide change in rs7041 or rs4588, Gc1f was assigned. If T changed to G at the rs7041 SNP position, Gc1s was assigned. If C changed to A at the rs4588 SNP position, Gc2 was assigned.
4. Statistical analysis
Total serum 25(OH)D, free 25(OH)D, calculated bioavailable 25(OH)D, free 25(OH)D, and VDBP concentrations were compared between patients with endometriosis and healthy controls using the independent-sample t-test. The Pearson chi-square test was used to analyze correlations between the GC genotype or allele and endometriosis. Statistical analysis was performed using Excel (Microsoft Corp., Redmond, WA, USA) and IBM SPSS ver. 21.0 (IBM Corp., Armonk, NY, USA).
Results
1. Demographic and clinical characteristics
A total of 32 women (16 with endometriosis and 16 healthy controls) were enrolled in this study. The demographic and clinical characteristics of the endometriosis and control groups are presented in Table 1. There were no statistically significant differences in age, gravidity, parity, body mass index (BMI), serum albumin levels, C-reactive protein (CRP) levels, or enrolled season between the endometriosis and control groups. However, serum CA-125 levels and the erythrocyte sedimentation rate (ESR) were significantly higher in the endometriosis patients than in the control group (p=0.004 and p=0.025, respectively).
2. Comparison of total serum, bioavailable, and free 25(OH)D concentrations between the endometriosis and control groups
The total serum, bioavailable, and free 25(OH)D VDBP concentrations of patients with endometriosis and healthy controls were compared (Figure 1). The median total 25(OH)D level was 9.55 ng/mL (interquartile range [IQR], 3.00–24.41 ng/mL) in patients with endometriosis and 16.48 ng/mL (IQR, 8.36–26.00 ng/mL) in healthy controls. The median bioavailable 25(OH)D level was 1.43 ng/mL (IQR, 0.89–4.50 ng/mL) in patients with endometriosis and 1.82 ng/mL (IQR, 1.03–4.42 ng/mL) in healthy controls. The median free 25(OH)D level was 4.32 pg/mL (IQR, 2.59–10.2 pg/mL) in patients with endometriosis and 4.67 pg/mL (IQR, 2.59–10.18 pg/mL) in healthy controls. The total serum 25(OH)D level was significantly lower in the endometriosis patients than in the healthy controls (p=0.017). However, serum bioavailable and free 25(OH)D levels were similar between the endometriosis and control groups (p=0.858 and p=0.961, respectively).
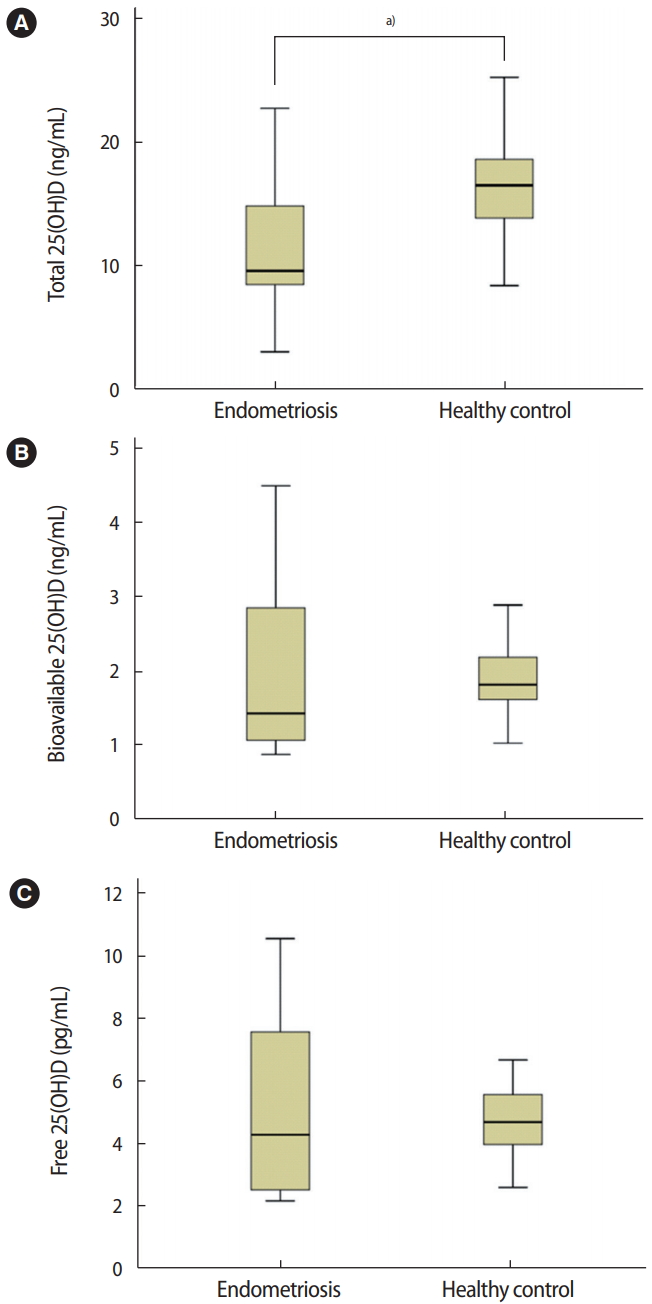
Comparison of serum total, bioavailable, and free 25-hydroxyl vitamin D (25(OH)D) concentrations between subjects with endometriosis and healthy controls. (A) Total 25(OH)D, a)p= 0.017. (B) Bioavailable 25(OH)D, p= 0.858. (C) Free 25(OH)D, p= 0.961. The independent sample t-test was used for the statistical analysis.
3. Analysis of serum VDBP levels in patients with endometriosis and healthy controls
The median serum VDBP concentrations in patients with endometriosis and healthy controls were 173.06 μg/mL (IQR, 76.39–265.08 μg/mL) and 158.58 μg/mL (IQR, 112.68–224.08 μg/mL), respectively (Figure 2). Serum VDBP levels did not differ significantly between the endometriosis and control groups (p=0.323).
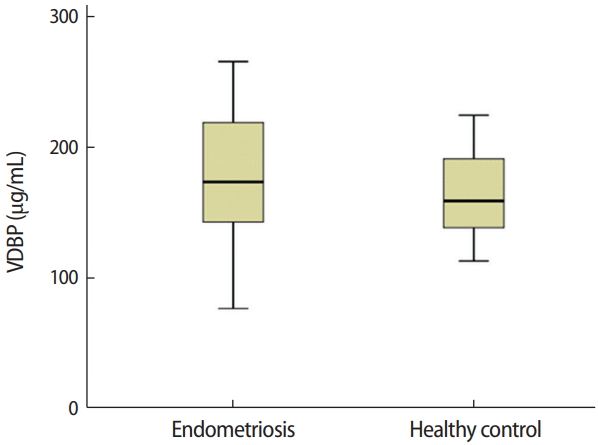
Comparison of serum vitamin D-binding protein (VDBP) concentrations in women with endometriosis and healthy controls (p= 0.323). The independent-sample t-test was used for the statistical analysis.
4. Analysis of genotype and allele frequencies of GC in patients with endometriosis and healthy controls
The genotype and allele frequencies of GC in patients with endometriosis and healthy controls are summarized in Table 2. The most frequent genotype in both groups was Gc1f/Gc1s (28.1%) and Gc1f/Gc2 (28.1%), followed by Gc2/Gc2 (15.6%). Among the three VDBP alleles, the most frequent allele was Gc1f (40.6%), followed by Gc2 (35.9%) and Gc1s (23.4%). The genotype and allele frequencies were similar between the two groups (p=0.788 and p=0.946, respectively).
Discussion
In this study, we analyzed serum vitamin D (total 25(OH)D, bioavailable 25(OH)D, and free 25(OH)D) levels, as well as VDBP gene polymorphisms, in Korean women with endometriosis. Our results demonstrated that Korean women with endometriosis had lower total serum 25(OH)D concentration than controls. However, there were no significant differences in serum bioavailable or free 25(OH)D levels between patients with endometriosis and healthy controls. In addition, serum VDBP concentrations and VDBP gene polymorphisms showed no significant association with endometriosis. To the best of our knowledge, this is the first study on VDBP polymorphisms in Korean women with endometriosis.
To elucidate the relationship between serum vitamin D levels and endometriosis, we enrolled Korean women with endometriosis (n=16) and an equal number of healthy women as controls (n=16) in this study. The clinical characteristics of the subjects enrolled in this study showed that age and BMI were similar between the two groups. In our study, CA125 concentrations were higher in patients with endometriosis than in the healthy controls. A meta-analysis of CA125 for the diagnosis of endometriosis suggested that serum CA125 levels might be a useful biomarker for the noninvasive diagnosis of endometriosis [29]. Our finding is consistent with this previous meta-analysis. In addition, the endometriosis patients included in our study had endometriosis confirmed pathologically postoperatively. In other words, these patients had severe endometriosis that required surgery. Thus, the CA-125 levels in the patients with endometriosis included in our study appeared to be higher than those in the cohorts of other studies.
While the CRP elevation in the endometriosis group was not statistically significant, the higher ESR observed in the endometriosis group did reflect a statistically significant difference. The reason for this finding might be that endometriosis can change from an acute to chronic process and be accompanied by tissue destruction, as in malignancy. The inflammatory marker CRP has been shown to be upregulated in women with endometriosis, especially when examined with a high-sensitivity assay, making it possible to detect subclinical inflammation [30]. However, other studies were not able to identify upregulation of CRP in women with endometriosis [31,32]. In a retrospective cohort study, discrepancies between CRP and the ESR have been reported. These discrepancies might have been due to timing of various studies, with a rise in CRP manifesting before the ESR elevates, or due to the possibility that the ESR does not change in response to minor inflammation [33]. Patients with a high ESR and normal CRP levels most likely have conditions without demonstrable systemic inflammation, such as malignancy.
In our study, both endometriosis and control groups had mean serum total 25(OH)D levels of less than 20 ng/mL. A previous epidemiological study by our study group reported that over 80% of women in our local region were vitamin D-deficient [34]. The results of the present study are consistent with those of the previous study [34]. However, in the present study, we first demonstrated that although most endometriosis patients and healthy controls had vitamin D deficiency, women with endometriosis had lower total serum 25(OH)D concentrations than the control group, suggesting that low levels of vitamin D could be associated with the pathogenesis of endometriosis. A previous study of the in vitro effects of vitamin D on human endometriotic cells also reported that vitamin D regulates inflammation and proliferation in endometriosis, suggesting that vitamin D could be used as a therapeutic strategy to manage endometriosis [35]. Supporting this proposal, vitamin D has been reported to be involved in the development and progression of endometriosis in animal experiments. For example, Abbas et al. [36] reported the regression of endometriotic implants treated with vitamin D3 in a rat model. Other reports have found low in vitro fertilization success rates when vitamin D concentrations are low in blood or follicular fluid [37]. These results are consistent with the expectation that pregnancy rates in patients with endometriosis will be low.
Although serum levels of bioavailable and free 25(OH)D were lower in the endometriosis patients, the difference between the endometriosis and control groups was not significant. The same tendency was observed for serum VDBP levels between the two groups. The limited sample size of our study might explain the lack of statistical significance in these results.
In our study, serum levels of VDBP did not differ significantly between the endometriosis and control groups. Borkowski et al. [38] found no significant difference in total VDBP concentrations in serum and peritoneal fluid between women with endometriosis and those without endometriosis. Using two-dimensional polyacrylamide gel electrophoresis, Ferrero et al. [39] found that one isoform of VDBP was present at significantly lower levels in the peritoneal fluid, but not in the plasma, of women with endometriosis than in controls. Another study of VDBP reported that the abundance of 25 protein spots, including acute-phase proteins and complement components, differed significantly between women with endometriosis and controls. VDBP protein levels were approximately three-fold higher in the endometriosis group than in the control group [24].
Polymorphisms located in genes encoding proteins mediated by vitamin D might be risk factors for endometriosis and infertility [22]. Numerous polymorphisms in VDBP and functional variants are known to affect vitamin D activity. Two well-known single nucleotide polymorphisms (SNPs)—rs7041 (NM_000583.3:c.1296T >G; NP_000574.2:p.Asp432Glu) and rs4588 (c.1307C>A; p.Thr436Lys)— are known to give rise to three major polymorphic isoforms of VDBP (Gc1F, Gc1S, and Gc2) [20]. In our study, Gc1f was the most frequent polymorphic allele in the endometriosis group (at 40.6%), consistent with a previous study on GC polymorphisms in various ethnic groups, which reported that the Gc1f allele was the most commonly inherited form and the most frequently reported polymorphic allele in Asian and African populations [40]. To our knowledge, research on GC polymorphisms is scarce and studies of GC polymorphisms in the Korean population are extremely rare. Faserl et al. [24] analyzed specific allele products using nanoscale liquid chromatography-electrospray ionization-mass spectrometry in women with endometriosis and found that the most prevalent VDBP Gc2 genotype in endometriosis may allow endometriotic tissues to implant in the peritoneal cavity. However, we did not find any correlation between the VDBP genotype and endometriosis in our study of Korean women. A study comparing 25(OH)D and VDBP concentrations in African-American and European-American women concluded that African-American women had lower concentrations of total 25(OH)D than did European-American women [41]. However, both groups had similar VDBP concentrations, suggesting that African-American women might have had lower concentrations of free 25(OH)D [41]. Although the demographic and lifestyle determinants of 25(OH)D concentrations were similar between the two groups in this study, genetic determinants may be ethnicity-specific. The relationship between VDBP genotype and endometriosis in our study was not statistically significant. However, the study was meaningful in that we studied the associations of VDBP gene polymorphisms with endometriosis in Korean women for the first time.
We acknowledge that our study has some limitations. First, the number of participants enrolled in our study was relatively small. Second, we did not have information regarding a food questionnaire to investigate dietary habits or levels of sun exposure. Third, our study did not stratify participants according to the severity of endometriosis. This needs to be overcome in further studies. Fourth, we did not use liquid chromatography-tandem mass spectrometry (LCMS/MS), a gold standard method, to measure total 25(OH)D levels. However, the Elecsys vitamin D total kit with the Cobas e602 module (Roche Diagnostics) used in this study has been previously evaluated and reported to be a comparable assay to the LC-MS/MS method [42]. An advantage of our study was that we measured serum levels of 25(OH)D and VDBP in healthy women and those with endometriosis. To the best of our knowledge, this is the first study to investigate the frequency of VDBP gene polymorphisms in Korean women with endometriosis.
In conclusion, Korean women with endometriosis had lower total serum concentrations of 25(OH)D than controls. Serum VDBP concentrations and VDBP gene polymorphisms (rs4588 and rs7041) had no significant association with endometriosis. Further studies are needed to investigate the pathophysiology and clinical implications of 25(OH)D and VDBP levels in endometriosis.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Author contributions
Conceptualization: MCC, WJC. Funding acquisition: MCC. Methodology: JHK, MHJ, IAC, HCJ, JKS, SAL, JHL. Writing - original draft: MCC. Writing - review & editing: WJC.


