Correlations between embryo morphokinetic development and maternal age: Results from an intracytoplasmic sperm injection program
Article information
Abstract
Objective
It is widely accepted that aging decreases women’s fertility capacity. The aim of this study was to assess correlations between maternal age and the morphokinetic parameters and cleavage pattern of embryos.
Methods
The morphokinetics of embryos derived from women <30, 30–35, 36–40, and >40 years of age were compared retrospectively in terms of time of second polar body extrusion, time of pronuclei appearance, time of pronuclei fading, and time of two to eight discrete cells (t2–t8). Furthermore, abnormal cleavage patterns such as uneven blastomeres at the two-cell stage, cell fusion (Fu), and trichotomous mitoses (TM) were assessed.
Results
Only t5 occurred later in women aged 36–40 and >40 years when compared with those aged <30 and 30–35 years (p<0.001). Other morphokinetic timing parameters, as well the presence of uneven blastomeres, were comparable between the groups (p>0.05). However, Fu and TM were more common in women aged >40 years than in younger women (p<0.001).
Conclusion
Maternal age was correlated with the cleavage pattern of embryos. Therefore, evaluating embryo morphokinetics may contribute to optimal embryo selection, thereby increasing fertility in patients with advanced maternal age.
Introduction
Approximately one-sixth of couples encounter infertility problems. However, assisted reproductive technology (ART) can help such couples conceive and give birth to healthy babies [1]. One of the most significant factors that influences ART outcomes is maternal age [2]. It is widely accepted that aging decreases women’s fertility capacity [3]. In recent decades, cultural and social trends have led women to postpone childbearing. Gleicher et al. [4] stated that older ART patients require more cycles and have a lower likelihood of pregnancy. The decrease in oocyte number and quality as a result of ovarian aging is the main cause for the poorer ART outcomes in patients with advanced maternal age [5]. However, it has been proposed that the decrease in fertility with age is due to oocyte aging, rather than the number of oocytes, changes in women’s endocrine profile, or decreasing endometrial receptivity [3]. Nonetheless, oocyte quality is an important factor that affects embryo quality, which is also an important factor influencing both pregnancy and live birth [4].
One of the main challenges in ART programs is the selection of embryos with the highest developmental competence for transfer. Although the conditions of clinical embryology laboratories have improved in recent years, difficulties remain in the conventional morphological assessment and selection of embryos based on discrete developmental information [6]. The development of embryos is dynamic, in that their morphology changes over time [7]. Furthermore, conventional assessments expose embryos to unstable conditions outside the incubator terms of light, temperature, and pH fluctuation [8].
In recent years, time-lapse monitoring (TLM) has been introduced, enabling the continuous monitoring of embryo growth while maintaining optimal culture conditions. This technology facilitates the analysis of kinetic parameters and cleavage patterns linked to higher developmental competence of embryos [9]. Therefore, TLM may enable optimal embryo selection in older women, which may improve their ART outcomes. To the best of our knowledge, few studies have reported the morphokinetics of embryos derived from women of different ages [10,11]. We assessed the morphokinetic parameters and cleavage patterns of embryos derived from women aged < 30, 30–35, 36–40, and > 40 years.
Methods
1. Study design
In this retrospective study, cumulus-oocyte complexes (COCs) were obtained from 73 infertile patients aged between 26 and 43 years (mean, 32.3 ± 8.1 years) undergoing oocyte retrieval for intracytoplasmic sperm injection (ICSI) at the Yazd Reproductive Sciences Institute. Couples with severe male factor infertility (sperm concentration, < 4 × 106 /mL) and those with surgically or frozen retrieved spermatozoa were excluded due to the poor recovery of motile spermatozoa. Normally fertilized oocytes were classified according to maternal age ( < 30, 30–35, 36–40, and > 40 years). The Ethics Committee of ethics committee of the Research and Clinical Center for Infertility, Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran, approved this study (No. IR.SSU.RSI.REC.1395.2).
2. Ovarian stimulation
The patients were stimulated with a standard gonadotropin-releasing hormone (GnRH) antagonist protocol. In the antagonist protocol, 150 IU/day of follicle-stimulating hormone (Gonal F; Serono, Geneva, Switzerland) was injected on day 2 of the menstrual cycle. When at least one follicle reached 14 mm, 0.25 mg of a GnRH antagonist (Cetrotide; Merck Serono, Darmstadt, Germany) was injected and continued until the day of human chorionic gonadotropin (hCG) injection. When at least two 17-mm follicles were seen on transvaginal ultrasound, recombinant hCG (Ovidrel, Serono) was administered to trigger final maturation and ovulation. Approximately 36 hours later, ultrasound-guided oocyte collection was done using a single-lumen aspiration needle (Wallace; Smiths Medical, Ashford, UK).
3. Oocytes preparation and injection
The collected oocytes were placed in in vitro fertilization medium (Vitrolife, Västra Frölunda, Sweden) and incubated at 37°C, under 6% CO2, 5% O2, and 89% N2 for 2–3 hours. The COCs were denuded of cumulus cells mechanically using glass pipettes and enzymatically with 80 IU/mL hyaluronidase (Sigma, Cream Ridge, NJ, USA). Then, MII oocytes using the husband’s prepared spermatozoa were injected through ICSI.
4. Embryo culture
After injection, the oocytes were individually cultured in a culture dish containing pre-equilibrated G-1 medium (Vitrolife) covered with sterile mineral oil and placed in the time-lapse microscope (Primo Vision Time-lapse Embryo Monitoring System, Goteborg, Sweden) in a triple-gas incubator for 3 days.
5. Time-lapse analysis
Every 10 minutes, images of each embryo were taken in seven focal planes. Only oocytes with normal fertilization (presence of two pronuclei and two polar bodies) were included in the study. One embryologist (AF) did all annotations. The morphokinetics of the embryos, including the time of the second polar body extrusion (tPB2), time of pronuclei appearance (tPNa), time of pronuclei fading (tPNf), and time of two to eight discrete cells (t2–t8), were monitored. In addition, the following abnormal cleavage patterns were analyzed: (1) uneven blastomeres at the two-cell stage where “uneven” was defined as a size difference of > 33% between the sibling blastomeres [12]; (2) cell fusion (Fu), in which the fusion or merging of blastomeres entails a reduction in the cell number of an embryo, giving it a reverse-cleavage appearance, or a blastomere is reabsorbed after cleavage; and (3) trichotomous mitoses (TM), in which a single blastomere divides directly from one to three cells.
6. Statistics
The normality of the distribution of the results was examined using the Kolmogorov-Smirnov test. One-way analysis of variance and the Kruskal-Wallis test were used to analyze the between-group differences for normally and non-normally distributed data, respectively. A logistic regression model was used analyze the independent effect of age on the cleavage pattern. Odds ratio and 95% confidence interval were calculated. The p-values < 0.05 were considered to indicate statistical significance. Statistical analysis was performed using IBM SPSS ver. 20.0 (IBM Corp., Armonk, NY, USA).
Results
A total of 499 viable zygotes were derived from 73 women, of which 129 zygotes were derived from women < 30 years of age, 126 from women 30–35 years of age, 139 of them from women 36–40 years of age, and 105 were derived from women > 40 years of age. No significant differences were found according to age group in demographic characteristics or basal hormonal status (Table 1).
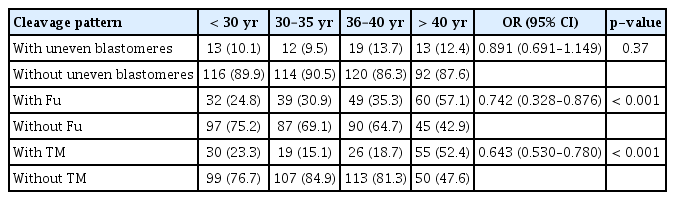
The time-lapse analysis showed that on average, t5 was significantly prolonged in older women (36–40 and > 40 years; 52.29 ± 8.03 hours and 53.02 ± 8.13 hours, respectively) compared to younger women (< 30 and 30–35 years; 45.84 ± 7.07 and 49.20 ± 7.71 hours, respectively) (p< 0.001). However, the timing of t5 was comparable between women aged 36–40, and > 40 years, as well as between those aged < 30 and 30–35 years (p> 0.05). Furthermore, the average timing of tPB2, tPNa, tPNf, t2, t3, t4, t6, t7, and t8 were comparable among the different age groups (p> 0.05) (Table 2). The frequency of uneven blastomeres was similar among women aged < 30, 30–35, 36–40 and > 40 years (10.1%, 9.5%, 13.7%, and 12.4%, respectively) (p> 0.05) (Table 3).
Our study also demonstrated that there was a significant correlation between the frequency of Fu embryos and women’s age ( < 30, 30–35, 36–40, and > 40 years: 24.8%, 30.9%, 35.3%, and 57.1%, respectively; p< 0.001). As shown in Table 3, there was a significant relationship between women’s age and the frequency of TM embryos (< 30, 30–35, 36–40, and > 40 years: 23.3%, 15.1%, 18.7%, and 52.4%, respectively; p< 0.001).
Discussion
This study was designed to compare the morphokinetics of embryos derived from women aged < 30, 30–35, 36–40, and > 40 years. Only t5 occurred later among women aged 36–40 and > 40 years than among those aged < 30 and 30–35 years. Other morphokinetic timing parameters and the frequency of uneven blastomeres were comparable. However, the morphokinetic parameters of Fu and TM in embryos increased with age. It is well known that the success of ART decreases noticeably with advanced maternal age [13]. Increased age in women causes a poor response to ovarian hyperstimulation, decreases the number of retrieved oocytes, and reduces the rates of fertilization, high-quality embryo formation, implantation, and pregnancy [14-16].
Yan et al. [17] showed that despite similar rates of two-pronuclear zygote and high-quality embryo formation, the clinical pregnancy rate was significantly lower in older infertile women. Recently, it has been reported that the morphokinetic parameters of implanted embryos were similar across different age groups [10]. In contrast, our study showed that only t5 occurred later in patients with advanced age. Previous studies compared morphokinetic parameters between different ages, whereas we assessed women aged < 30, 30–35, 36– 40, and > 40 years old. This could explain the inconsistency between our findings and those of the aforementioned studies. Another study reported that women’s age was not associated with the timing of embryo cleavage [18]. Unlike our study, they surveyed only 32- and 35-year-old women.
However, Akhter and Shahab [11] showed that women’s age was associated with differences in the timing of embryo cleavage. It has been reported that the reduction in fertility with advanced age is largely correlated to low oocyte quality, due to patterns of aging in the ovary. The mechanisms through which oocytes age in response to aging of the ovary are not obvious, unlike the mechanisms of postovulatory oocyte aging [19]. Advanced maternal age induces a rate of oocyte of aneuploidy of nearly 50%, which may be due to low cohesin levels. Cohesin plays a role in stabilizing the chiasmata and holding the sister chromatids tightly together [20]. In addition, reduced levels of cohesin may cause errors in meiotic segregation in oocytes [21]. Recently, Tsutsumi et al. [21] reported that the aging process affected changes in the structure of chromatin and gene expression, as well as epigenetic alterations due to methyltransferases and demethylases.
The oocyte itself plays an influential role in embryo development. Half of the embryo’s chromosomes are delivered by the oocyte, as well as the mitochondrial genome, which may be affected by aging [22]. In our recent studies, the quality and maturity of oocytes were found to affect the morphokinetics of derived embryos [23-26]. Since the introduction of TLM, improved ART outcomes have been observed via the continuous and uninterrupted culture of human embryos. However, the selection of embryos with the highest implantation competence through an assessment of morphokinetics relies largely on the timing of cell divisions. Early morphokinetic parameters are positively associated with embryo developmental competence [7]. A hierarchical model of embryo selection was developed based on t5, the second synchronization (time between t3 to t4), and the second cell cycle (time between t2 to t3). The rates of both implantation and blastocyst formation were increased by using this embryo selection model. In this hierarchical model, t5 is the first important criterion [27]. Other studies have also reported that t5 was a main predictor of high-quality blastocysts [28,29]. In addition to morphokinetic parameters, other important phenomena may only be observed using TLM. These may help optimize embryo selection [30]. Abnormal cleavage patterns, such as uneven blastomeres, Fu, and TM are impossible to see using conventional embryo assessment techniques [31]. Several studies have shown that embryos with uneven blastomeres, TM, and Fu showed worse ART outcomes [27,32,33]. The molecular mechanism of these phenomena are not clear, but mitotic errors may play a major role [34]. Desai et al. [35] reported that the presence of an abnormal cleavage pattern (including uneven blastomeres, TM, and Fu) in addition to other cleavage anomalies was associated with euploidy and, in some cases, decreased developmental potential.
In conclusion, correlations were observed between embryo cleavage patterns and advanced maternal age. Therefore, evaluating embryo morphokinetics may help optimize embryo selection and improve ART outcomes in patients with advanced maternal age. However, a limitation of this study is that it did not investigate blastocyst formation or implantation and pregnancy outcomes. Larger studies on embryo morphokinetics and clinical outcomes are warranted to validate the findings of the present study.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Author contributions
Conceptualization: MAK. Data curation: AF. Formal analysis: EM. Funding acquisition: MAK. Methodology: AF. Project administration: MAK. Visualization: AF. Writing - original draft: AF. Writing - review & editing: EM.
Acknowledgements
The authors would like to thank the colleagues (Research and Clinical Center for Infertility, Yazd Reproductive Sciences Institute) who helped with data collection.