An effective method for improving outcomes in patients with a fertilization defect
Article information
Abstract
The effect of artificial oocyte activation (AOA) with a calcium ionophore on intracytoplasmic morphologically selected sperm injection (IMSI) was examined in patients with histories of repeated failed implantation attempts. Four singleton pregnancies and one twin pregnancy were obtained after embryos transfer (5/14, 35.7%). Therefore, AOA combined with IMSI can be considered an option for cycles with a fertilization defect and recurrent implantation failures.
Recurrent implantation failure refers to failures of embryo implantation following at least two embryo transfers. An appropriate application of fertilization methods for individual patients is a crucial step in conception via the use of artificial reproductive technologies (ARTs). In particular, intracytoplasmic sperm injection (ICSI) has been commonly used as a fertilization method in ART cycles to overcome severe male infertility. However, post-ICSI fertilization failure can be attributed to a lack of oocyte activation, and the assistance of oocyte activation with a calcium ionophore following ICSI has been proposed to solve this problem [1]. Following treatment with calcium ionophore, the number of Ca2+ ions in the oocyte cell membrane increases and the extracellular Ca2+ ions flow into the oocyte. Finally, the calcium ionophore induces the Ca2+ concentration peak.
It is clear that performing ICSI with abnormal sperm can potentially induce paternal effects and that chromosomally abnormal sperm have an adverse effect on the outcomes [2]. Intracytoplasmic morphologically selected sperm injection (IMSI) represents an alternative for patients who have experienced repeated failed ICSI cycles. Further, the fine morphological state of the sperm nucleus has been shown to be an important factor in achieving pregnancy after ICSI [3].
This study was designed to analyze whether artificial oocyte activation (AOA) was associated with the results of the IMSI treatment in patients with zero or low fertilization rates despite AOA being performed after ICSI to overcome the difficulties of the fertilization procedure.
The AOAs combined with IMSIs were performed between January 2012 and July 2013. This retrospective study was approved by the Institutional Review Board of Ethics and Research (2013-008). A total of 14 patients (35.57±3.90 years) who underwent IMSI-AOA were included in this study. The criteria for inclusion in this study were as follows: 1) male-factor-mediated infertility with oligo-astheno-teratozoospermia (OAT); 2) an experience of a zero or low (<35%) fertilization rate following ICSI-AOA; 3) a day-3 follicular stimulating hormone level below 10 IU/L and an AMH above 1 ng/mL; and 4) a body mass index of between 19 kg/m2 and 25 kg/m2.
All patients underwent standard controlled ovarian stimulation (COS) via the administration of gonadotropins by using a long stimulation with agonist cycles. The zygotes were cultured in a COOK medium (Brisbane, Australia). Three days after the insemination, the embryos were transferred into the uterus of the female patient.
Serum HCG levels were measured 14 days after the oocytes were transplanted back into the uterus, and clinical pregnancies were confirmed by vaginal ultrasound scans that revealed the presence of an intrauterine gestational sac 6-7 weeks after embryo transfer.
Normal spermatozoa were confirmed when the shapes of the nuclei were smooth and symmetrical with an ovular configuration, and the average lengths and widths were 4.75±0.28 µm and 3.28±0.20 µm, respectively [4]. Additionally, the selected nuclei contained no more than one vacuole that comprised less than 4% of the nuclear area (Figure 1). All motile spermatozoa were analyzed using a high-magnification (6,000×) microscope (TE 2000 U, Nikon, Tokyo, Japan) with 60× air objectives and modulation contrast illumination (RI IMSI, RI, Falmouth, Cornwall, UK). After the selection of normal spermatozoa by high magnification, the oocytes underwent ICSI within 2 hours of oocyte denudation. Fertilization was confirmed 17-19 hours after insemination.

Spermatozoa observed under high magnification (6,000×) microscopy with a 60× air objective with modulation contrast. (A) A normal spermatozoon, (B) a spermatozoon with two large vacuoles (arrow), and (C) a spermatozoon with a large vacuole (arrow).
The calcium ionophore A23187 (Sigma Chemical, Sigma, St Louis, MO, USA) was used for AOA, as previously described [5]. The final solution (10 µmol/L) was prepared prior to ICSI or IMSI. Thirty minutes after insemination, the oocytes were exposed to the calcium ionophore for 30 minutes at 37℃ in 6% CO2 and 5% O2.
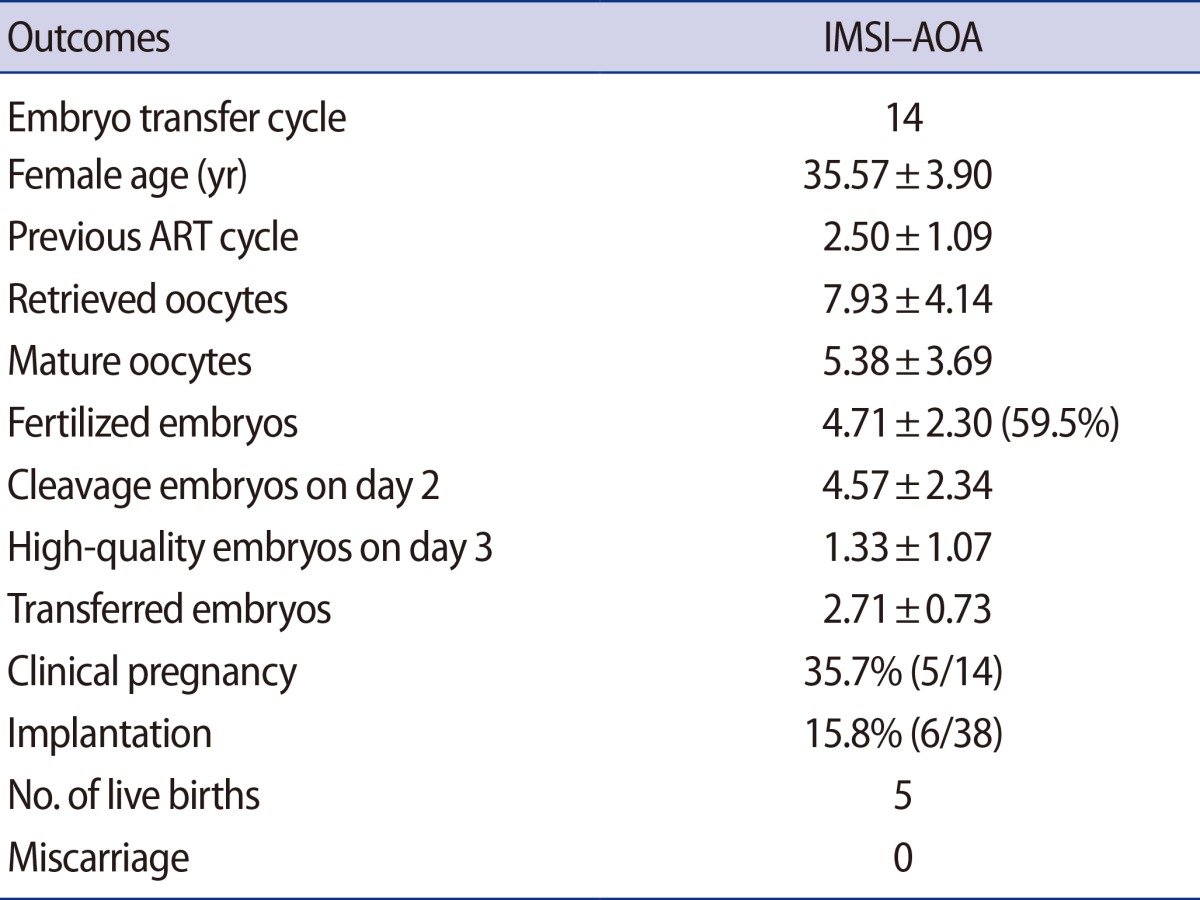
AOA with a calcium ionophore was performed in 14 IMSI cycles with histories of zero or low fertilization rates in the previous AOA following ICSI (Table 1). The average age (±SD) of the females was 35.57±3.90, and the mean number of IVF experiences per patient was 2.50±1.09. The average number of oocytes retrieved was 7.93, and on average, 5.38 mature oocytes were used for ICSI. The fertilization rate was 62.9% (4.71±2.30), and the mean number (±SD) of embryos that developed normally on the day after the confirmation of fertilization was 4.57±2.34. A mean of 2.71±0.73 embryos per patient were transferred. After embryo transfer, the clinical pregnancy rate was 35.7%, and four singleton pregnancies and one twin pregnancy were obtained. These five pregnancies produced six healthy babies.
In a preliminary study, increased fertilization rates and significantly higher clinical pregnancy and implantation rates were achieved using AOA following ICSI compared with the previous treatment with standard ICSI in a group of patients who had experienced total fertilization failure or low fertilization rates (<50%) in their previous ICSI cycles. Furthermore, 38 healthy babies were delivered from 185 ICSI cycles by using AOA with a calcium ion [5]. These results support the safety of AOA with a calcium ionophore and suggest the effectiveness of AOA for ICSI cycles in patients with previous experiences of zero or low fertilization rates.
However, although these results indicate that the application of AOA for ICSI cycles in which defects in the fertilization procedure occurred in the previous ICSI cycles is clinical beneficial, the performance of AOA does not always result in appropriate fertilization. There are important aspects related to an appropriate selection of fine spermatozoa and oocyte activation. Therefore, it is crucial to establish a novel technique that can overcome difficulties in such cycles prior to subsequent IVF treatments.
It has recently been demonstrated that the selection of high-quality sperm is important, and this selection depends on features such as motility, morphology, and intact chromosomes. Conventional ICSI is performed after the morphological selection of spermatozoa under magnification of 200× to 400×, which enables the detection of the defects of the head, neck, and tail. However, this technique is of limited utility for investigating nuclear vacuoles in the sperm head. A significant correlation between the incidence of morphologically normal spermatozoa without vacuoles in the head [6] and higher fertilization using IMSI has been reported [4,7]. The significant benefits of the application of IMSI have been demonstrated to result in higher ongoing-pregnancy rates among patients with at least two prior implantation failures [8] and among all patients with severe OAT male-factor infertility [9].
This center began the application of IMSI for patients who failed IVF treatment at least twice following fertilization by ICSI. There were no statistically significant differences in the fertilization rate, but the clinical pregnancy rate and implantation rate were higher than those of the previous ICSI cycles in the same couples [10]. Calcium ionophores have been used at this center to overcome fertilization failure in cases with histories of low fertilization rates after ICSI. Although the oocytes were activated artificially after ICSI, some cycles did not exhibit sufficient fertilization rates. To rescue such cycles, IMSI was applied in combination with AOA following the acquisition of written informed consent to share the outcomes for research purposes from all the patients.
The data from this combination of IMSI and AOA indicate that sperm selection under high magnification might serve a protective role that enables one to avoid the detrimental effects of inadequate sperm. However, this study has a limitation: The number of patients examined in the study of the relationships between AOA combined with IMSI and the clinical outcomes was small. Therefore, a well-designed large-scale study is needed.
In conclusion, AOA with a calcium ionophore combined with IMSI might be a good technique to improve the clinical outcomes of couples with histories of zero or low fertilization rates following ICSI-AOA. This option could be particularly relevant to couples with histories of repeated implantation failures.
Notes
No potential conflict of interest relevant to this article was reported.