Developmental competence of in vitro-matured human oocytes obtained from pregnant and non-pregnant women
Article information
Abstract
Objective
The aim of this study was to compare the rate of maturation, fertilization, and embryo development of in vitro-matured human oocytes derived from pregnant and non-pregnant women.
Methods
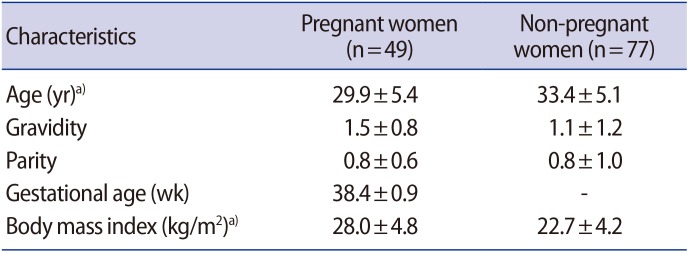
Immature oocytes were obtained by needle aspiration from 49 pregnant women (group 1) who underwent a cesarean section at term and 77 non-pregnant women (group 2) who underwent a gynecological operation during the same period (8 months). Healthy immature oocytes (530 in group 1 and 539 in group 2) were cultured and assessed for maturation 36 hours later. Mature oocytes were inseminated by intracytoplasmic sperm injection and cultured up to 144 hours.
Results
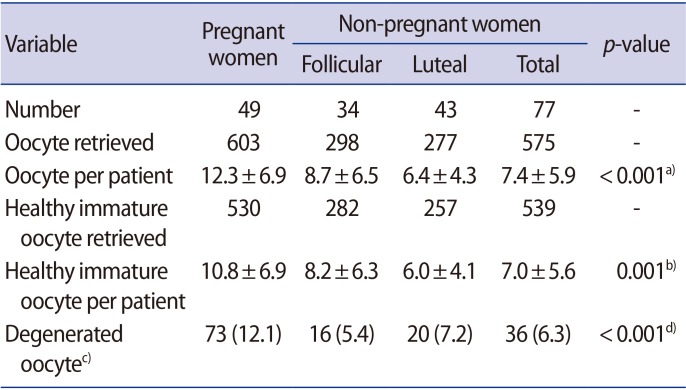
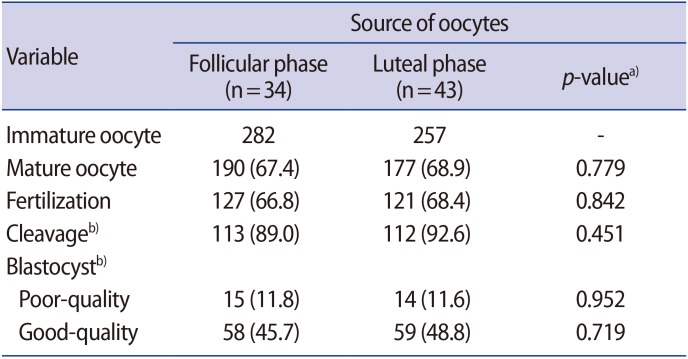
The percentage of degenerated oocytes was significantly higher (12.1% vs. 6.3%; p<0.001) in group 1 than in group 2. There was no significant difference in the maturation rate (66.8% vs. 68.1%; p=0.698), fertilization rate (66.7% vs. 67.6%; p=0.857), or the rate of formation of good-quality blastocysts (46.2% vs. 47.2%; p=0.898) in oocytes obtained from pregnant and non-pregnant women.
Conclusion
The developmental competence of immature oocytes did not differ between pregnant and non-pregnant women.
Introduction
Conventional oocyte donation involves controlled ovarian stimulation, followed by oocyte retrieval. Both procedures impose additional risks that may not be necessary, making it difficult to find non-commercial donors. A potential alternative source could be immature oocytes obtained during a routine gynecological operation or cesarean section, followed by in vitro maturation (IVM). Previous studies showed that this procedure added 10 minutes or less to the operating time and exposed the donors to minimal additional risks [12]. The use of such donors would simplify the synchronization of recipients in anticipation for the date of the operation. Collection of immature oocytes during a cesarean delivery is also a promising option for those who are pregnant from a previous in vitro fertilization (IVF) treatment and desire future treatment despite not having any remaining cryopreserved oocytes/embryos [2].
In an animal study, Behboodi et al. [3] found no differences in the maturation, cleavage, blastocyst formation, or hatching rates of blastocysts derived from oocytes of pregnant and non-pregnant cows. In contrast, Gambini et al. [4] reported that pregnant mare oocytes showed a lower maturation rate than those from non-pregnant animals, but found no differences in cleavage or blastocyst development. Conversely, Torner et al. [5] observed more rapid maturation in immature oocytes from non-pregnant camels than in those from pregnant camels. In a primate study, Brzyski et al. [6] obtained a good IVM rate for baboon oocytes retrieved during a cesarean operation. Xu et al. [7] obtained similar maturation and developmental rates to the morula stage for baboon oocytes obtained in the proliferative and luteal phases.
In humans, controversy similarly exists regarding the developmental competence of oocytes obtained from pregnant and non-pregnant women. Cha et al. [8] reported the first successful birth from IVM oocytes obtained from a patient with polycystic ovaries. Subsequent reports mainly focused on the successful use of immature oocytes from non-pregnant women [9101112], although there have been a few reports of pregnancies from IVM of oocytes retrieved during cesarean delivery [131415]. The purpose of this study was, therefore, to compare the maturation, fertilization, and embryo development of human IVM oocytes obtained during cesarean and gynecological operations in a parallel study.
Methods
This research was conducted at the Department of Obstetrics and Gynecology, Buddhachinaraj Hospital, and supported by the University Endowment Fund for Medical Research. The Research Ethics Committee of the Buddhachinaraj Hospital Medical School approved this study (No. 85/57). All participants provided written informed consent to participate in the study.
1. Subjects
Pregnant women who underwent cesarean deliveries at Buddhachinaraj Hospital Medical School were invited to participate in the study if they fulfilled the following inclusion criteria: (1) age 18–40 years; (2) a spontaneous singleton pregnancy; (3) a gestational age of 37–40 weeks; and (4) elective repeated or emergency cesarean section not due to fetal distress. Women scheduled for elective gynecological operations were invited to participate in the study if they were 18–40 years old and had not used any hormones for 3 months prior to surgery. In both groups, patients were excluded if they (1) had chronic diseases or medical or obstetric complications that could jeopardize their health by prolonging anesthesia or surgery, (2) could not read, write, or understand the Thai language, or (3) had excessive blood loss or unstable vital signs during the operation.
2. Oocyte collection
Cesarean or gynecological operations were performed under general anesthesia or spinal block. All visible follicles were aspirated with a 22-gauge needle connected to a 5-mL syringe, filled with 1 mL of warmed flushing medium (Ferticult Flushing Medium, FertiPro, Beernem, Belgium) after closure of the uterine wound or before performing gynecological surgery.
3. In vitro maturation
Follicular fluid was examined carefully under a stereomicroscope to identify immature oocyte-cumulus complexes (OCCs), which were small and dense. Only healthy-appearing oocytes, with multilayered or sparse cumulus, were used in this study. They were washed with oocyte washing medium, and transferred into SAGE oocyte maturation medium (Cooper Surgical, Trumbull, CT, USA), and cultured in groups of up to three in 25-µL drops of maturation medium, supplemented with 75 IU/L of human menopausal gonadotropin, as recommended by the manufacturer, under paraffin oil at 37℃ in a humidified atmosphere of 6% CO2 in air. The oocyte maturation medium was prepared 1 day before use and left to equilibrate overnight in the 6% CO2 incubator.
4. Fertilization and embryo development
The maturity of the oocytes was assessed 36 hours post-retrieval, after denudation of their cumulus cells by brief exposure to hyaluronidase. Mature oocytes were inseminated immediately by intracytoplasmic sperm injection (ICSI) to prevent oocyte aging, using spermatozoa donated for research. Following ICSI, oocytes were cultured in groups of up to three in 20-µL drops of embryo maintenance medium under paraffin oil. Micro-drops (25 µL) of embryo maintenance medium were prepared in 35×10-mm culture dishes (Falcon, Corning Life Sciences, Corning, NY, USA), and left to incubate overnight in a CO2 incubator 1 day before use, as recommended by the manufacturer.
Fertilization was assessed 18 hours after ICSI. After 72 hours of culture, the embryos were transferred into blastocyst medium, and cultured for another 72 hours. Embryos were examined once every 24 hours. Only fully expanded blastocysts and hatching or hatched blastocysts, containing a distinct inner cell mass and trophectoderm layer (grade 322 or better according to the Istanbul Consensus Scoring System) [16], were considered to be good-quality blastocysts.
5. Statistical analysis
Stata ver. 11 (StataCorp., College Station, TX, USA) was used for all statistical analyses. Comparisons of frequency data between groups, such as fertilization and cleavage rates, were performed using the chi-square test. Means were compared using the non-paired t-test or analysis of variance. A p-value below 0.05 was considered to indicate statistical significance.
Given a type I error of 5% (two-tailed) and a type II error of 20%, sample size calculation showed that at least 400 oocytes were required in each arm to demonstrate a 10% difference in the maturation rate between oocytes from pregnant and non-pregnant donors.
Results
Forty-nine pregnant women and 77 gynecological patients volunteered to participate in the study. Their characteristics are shown in Table 1. The indications for surgery were: symptomatic myoma uteri (34 cases), ovarian cyst (12 cases), endometriosis (15 cases), tubal sterilization (11 cases), and polycystic ovary syndrome not responding to clomiphene citrate (five cases). Detailed information about the harvested oocytes is shown in Table 2. The number of follicles per square centimeter at the time of aspiration was found to be significantly higher on the ovarian surface during late pregnancy than during the menstrual cycle (7.5±5.2 vs. 4.3±3.5 follicle/cm2; non-paired t-test, p<0.001).
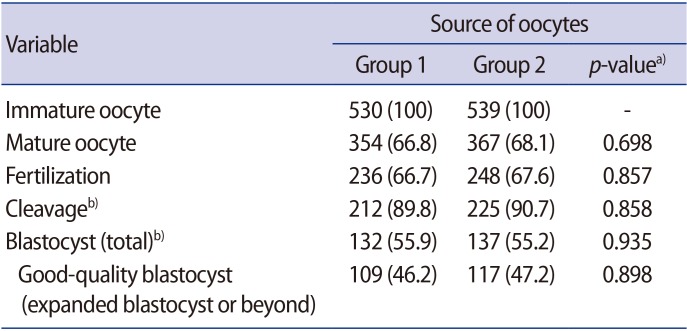
A total of 1,069 healthy immature oocytes were included in this study. There was no statistical difference in the IVM rate, fertilization, further cleavage, or blastocyst formation rate among the two groups (Table 3). Representative images of OCCs, IVM oocytes, and resulting embryos from pregnant and non-pregnant women are shown in Figures 1 and 2, respectively.
In vitro maturation and developmental competence of immature oocytes from pregnant (group 1) and non-pregnant women (group 2)
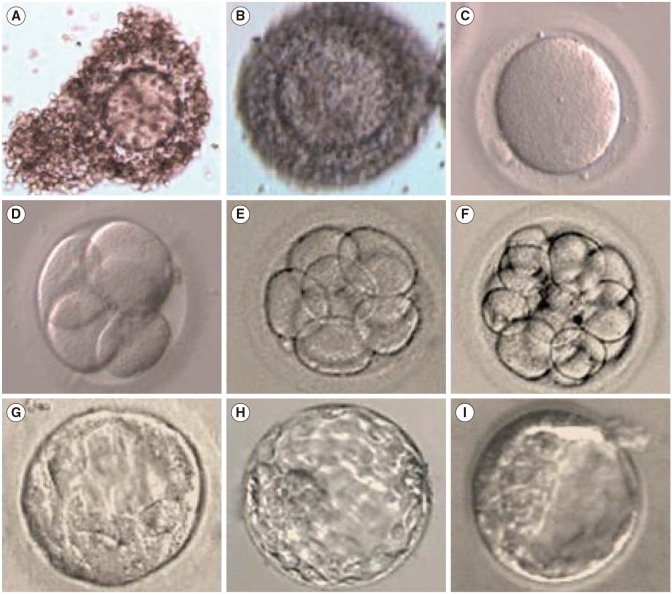
Representative images of oocyte-cumulus complexes, in vitro-matured oocytes, and resulting embryos from pregnant women: (A, B) immature oocytes with multilayered cumulus cells, (C) metaphase II (mature oocytes), (D) four-cell embryo, (E) eight-cell embryo, (F) morula, (G–I) blastocysts. Magnification of all panels, ×400.
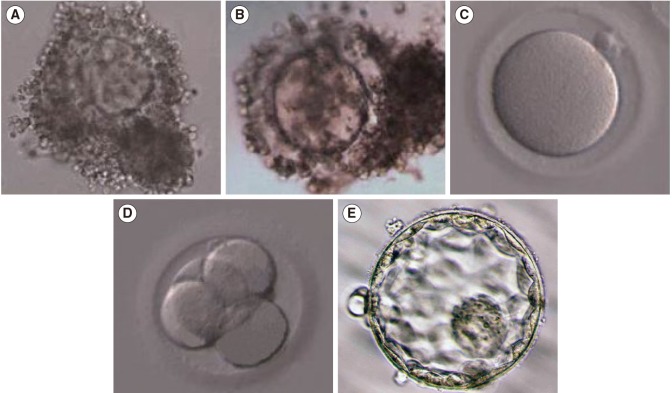
Representative images of oocyte-cumulus complexes, in vitro-matured oocytes, and resulting embryos from non-pregnant women: (A, B) immature oocytes with multilayered cumulus cells, (C) metaphase II (mature oocytes), (D) four-cell embryo, (E) blastocysts. Magnification of all panels, ×400.
We collected 282 oocytes from non-pregnant women during the follicular phase and 257 during the luteal phase. There were no significant differences between the groups in the maturation rate, fertilization rate, or rate of formation of good-quality blastocysts (Table 4).
Discussion
In humans, dominant follicle selection occurs around day 5 of the cycle. The selected follicle continues to develop, while non-selected follicles stop growing and undergo atresia [17]. Although granulosa cells of non-selected follicles show degenerative changes, the oocytes inside are still viable and competent for more than 1 week after follicle selection [18]. This is compatible with our finding that immature oocytes collected at any time before ovulation were developmentally competent and capable of developing into good-quality blastocysts after IVM and fertilization. A recent study confirmed that blastocysts that developed from fertilized IVM oocytes from small non-selected follicles were as competent as those that developed from dominant follicles in terms of pregnancy [18]. The recovery of healthy oocytes in the luteal phase, in this and other studies [192021], supports the concept that these oocytes came from newly developed follicles that emerged in a wave of luteal recruitment. Based on this current concept, many IVF programs now perform mild stimulation in the follicular phase in poor responders, followed by a second stimulation after oocyte retrieval (double- or dual-stimulation). This method takes advantage of follicular wave emergence in both phases of the menstrual cycle and enables oocyte accumulation in a shorter time frame [2223].
Follicular development has also been observed throughout various stages of human pregnancy [2425]. The concentrations of steroid hormones in antral follicles during late gestation were found to be almost the same as those in follicles of similar size from non-pregnant women, except for a significantly higher level of progesterone and a slightly lower level of follicle-stimulating hormone (FSH) [26]. This suggests that the regulatory mechanisms of small follicles could be similar during the menstrual cycle and pregnancy [242526]. The higher follicle density in pregnant women in our study could have been due to bias, as their average age was lower than that of the non-pregnant women. Moreover, patients who came for gynecological operations for ovarian cysts and endometriosis could have had a lower antral follicle count than pregnant women of the same age. The high percentage of oocyte degeneration in the pregnant group could possibly have been due to prolonged exposure to high progesterone and low FSH levels. These findings should be confirmed and elucidated in future studies.
To date, few reports have investigated IVM of immature oocytes collected during the follicular and luteal phases. One study found that luteal oocytes had a significantly higher maturation rate than those retrieved during the follicular phase [27], while another study found slightly lower maturation rates, but a trend towards a higher fertilization rate [28]. In this study, we obtained comparable yields between follicular- and luteal-phase oocytes, in accordance with another recent study [29]. The weakness of our study was that it was a prospective parallel study, not a randomized controlled trial, and the two groups might not have been completely comparable in terms of baseline characteristics.
In conclusion, immature oocytes retrieved from pregnant and non-pregnant women were able to undergo IVM, were fertilized, and gave rise to good-quality embryos in comparable yields. As these oocytes can be collected in any phase of the menstrual cycle without hormonal stimulation, and even during pregnancy, this method has a potential to serve as a promising source of oocytes for donation, fertility preservation, and stem cell research. Further studies are needed to define their implantation potential and future clinical use.
Acknowledgments
The authors would like to thank Buddhachinaraj Hospital Medical School for funding and for providing a professional English reviewer, Mr. Robert Moore, to help edit the manuscript.
Notes
This study was supported by the University Endowment Fund for Medical Research.
Conflict of interest: No potential conflict of interest relevant to this article was reported.