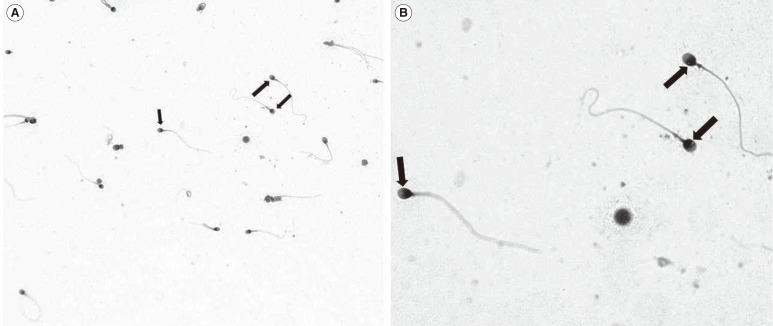
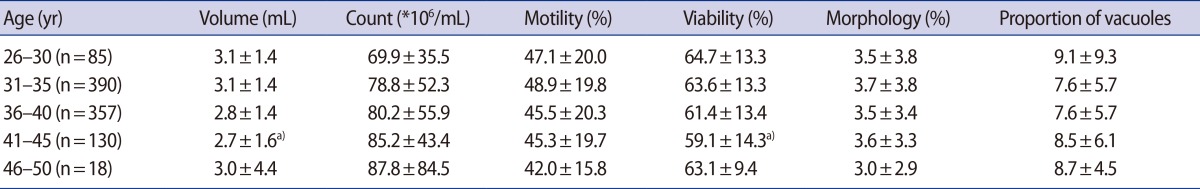
Observation of sperm-head vacuoles and sperm morphology under light microscope
Article information
Abstract
Objective
The presence of sperm-head vacuoles has been suspected to be deleterious to the outcomes of assisted reproductive technology (ART). It is difficult to accurately distinguish morphologically abnormal sperm with vacuoles under a light microscope. This study was performed to analyze the result of the observation of sperm-head vacuoles using Papanicolaou staining under a light microscope and whether the male partner's age affects these vacuoles.
Methods
Sperm morphology with vacuoles was evaluated using Papanicolaou staining and observed under a light microscope (400×) in 980 men. The normal morphology was divided into three categories (group A, <4% of normal morphology; group B, 4%-14% of normal morphology; and group C, >14% of normal morphology). The criteria for the sperm-head vacuoles were those given in the World Health Organization manual. For the analysis of the age factor, the participants were divided into the following groups: 26-30 years, 31-35 years, 36-40 years, 41-45 years, and 46-50 years.
Results
The percentage of sperm-head vacuoles increased with normal sperm morphology (group A vs. groups B, C) (p<0.05). In the case of the age factor, a statistically significant difference was not observed across any of the age groups.
Conclusion
A majority of the sperm-head vacuoles showed a statistically significant difference among normal morphology groups. Therefore, we should consider the probability of the percentage of sperm-head vacuoles not increasing with age but with abnormal sperm morphology. A further study is required to clarify the effect of the sperm-head vacuoles on ART outcomes.
Introduction
Semen analysis provides comprehensive information of the reproductive function of a male patient. It includes assessments of the sperm count, motility, viability, and morphology. The sperm count and motility patterns can be objectively examined using a computer-assisted sperm analyzer (CASA) system. Recently, the use of Kruger's strict criteria for the analysis of the sperm morphology has become the new standard in this field [1,2,3,4,5]. However, the analysis of sperm morphology is subjective and particularly difficult to standardize; in fact, a number of difficulties related to the lack of objectivity including a variation in interpretation have already been reported [6].
Normal spermatozoa have been defined, and sperm-head vacuoles have been described as a new abnormality [7,8]. Vacuoles in the sperm head have been reported to be associated with DNA fragmentation [9,10,11], aneuploidy [12,13], and chromatin defects [14,15,16]. However, the origin of these vacuoles is still debated [9,10,12,13,14,16,17,18].
The presence of large vacuoles in the sperm head has been suspected to have deleterious effects on the outcomes of assisted reproductive technology (ART) [8,11,19,20,21]. To clearly identify a vacuole in the sperm head, high-magnification interference contrast microscopy or motile sperm organelle morphology examination (MSOME) has been used widely. These techniques are used for optimal sperm selection before intracytoplasmic morphologically selected sperm injection (IMSI) [22]. However, many laboratories observed sperm morphology during ICSI under an inverted microscope without high-magnification microscopy (6,000×), which makes it impossible to accurately distinguish the morphologically abnormal spermatozoa because sperm-head vacuoles are not visible upon standard magnification.
The purpose of this study was to analyze the result of the observation of the sperm morphology with sperm-head vacuoles by using modified Papanicolaou staining under a light microscope (400×) during routine semen analysis and to investigate whether the male partner's age affects these vacuoles.
Methods
1. Patient characteristics and semen analysis
Semen quality was assessed for all the 980 men examined. The mean age of the patients was 36.0±4.3 years. Semen was collected by masturbation into a sterile plastic cup after a period of 3-5 days of sexual abstinence. For the semen analysis, the semen specimen was left for at least 30 minutes at room temperature for liquefaction. The sperm characteristics (volume, count, motility, viability, and morphology) and the criteria for the sperm-head vacuoles were assessed using the World Health Organization (WHO) guidelines [6]. The sperm morphology was evaluated using the modified Papanicolaou staining method under a light microscope (400×). The classification used considered all borderline forms abnormal morphology. The criteria for the sperm-head vacuoles were as follows: the acrosomal region should contain no large vacuoles, not occupy more than 20% of the sperm head, and have no more than two small vacuoles. The post-acrosomal region should not contain any vacuoles. One of these criteria of vacuoles was observed and was considered the sperm-head vacuole (Figure 1). For a comparison of the proportion of vacuoles in the sperm head, the sperm morphology was divided into three groups (group A, less than 4% of normal morphology; group B, 4%-14% of normal morphology; and group C, greater than 14% of normal morphology). Further, for the analysis of the age factor, patients were divided into the following age categories: 26-30 years, 31-35 years, 36-40 years, 41-45 years, and 46-50 years.
2. Statistical analysis
Statistical analysis was performed using the Statistical Package for the Social Sciences, ver. 12.0 (SPSS Inc., Chicago, IL, USA). Each parameter was compared among the groups by using one-way analysis of variance. A value of p<0.05 was considered statistically significant.
Results
The overall results of the conventional semen analysis were as follows: the semen volume was 3.0±1.6 mL, sperm count was 79.4±51.4×106/mL, sperm motility was 47.0%±19.9%, viability was 62.3%±13.5%, normal sperm morphology was 3.6%±3.5%, and proportion of vacuole was 7.9%±6.1%. The results of the sperm parameters and the sperm-head vacuoles showed a statistically significant difference between group A and groups B, C (p<0.05) (Table 1). In particular, the percentage of sperm-head vacuoles increased with the percentage of normal sperm morphology. Further, we observed the influence of male age on the semen quality and the sperm-head vacuoles (Table 2). Sperm morphology and the percentage of sperm-head vacuoles did not differ among the age groups, but semen volume and viability showed a statistically significant difference in the age group of 41-45 years (p<0.05). In the case of the age factor, a statistically significant difference was not observed in normal sperm morphology and sperm-head vacuoles across any of the five groups.
Discussion
For the evaluation of sperm morphology, Kruger et al. [23] reported the use of a modified method and the criteria used are referred to as the strict criteria or Kruger's strict criteria. On the basis of these criteria, it has been established that more than 14% of normal sperm morphology is a good predictive indicator of fertilization [1], pregnancy rates [2], and sperm function test [24,25].
Recently, sperm-head vacuoles have been described as a new abnormality [7,8] in male infertility. The proportion of these vacuoles is known to increase with an increase in the percentage of abnormal sperm morphology [13,26,27,28,29]. Many reports have shown that a nuclear origin and the presence of large vacuoles are associated with an increase in the DNA fragmentation [9,10,12]. However, the other data are in agreement with chromatin condensation defects [13,14,16] and are related to an increase in the aneuploidy rates [13]. The morphologically abnormal sperm and the presence of nuclear vacuoles seemed to have a negative impact on fertilization, embryo quality [19,30,31,32], and the later stages of zygote development in the ICSI cycles [8,20,29,33,34].
Further, the percentage of spermatozoa with large nuclear vacuoles (LNVs) significantly increased with a deterioration of the semen quality. A number of LNVs exhibited a significant negative correlation with the above mentioned sperm parameters in routine semen analysis [35]. However, many laboratories have observed sperm morphology during ICSI under an inverted microscope without a high magnification (6,000×) makes it impossible to accurately distinguish the morphologically abnormal spermatozoa under an inverted microscope with standard magnification. For the observation of sperm morphology, some staining methods (Papanicolaou stain, Shorr stain, or Diff-Quik stain) are recommended, and recently, the range of normal sperm morphology was changed to 4% [6]. A modified Papanicolaou staining method gives good staining of spermatozoa and other cells [6]. It stains pale blue in the acrosomal region and dark blue in the post-acrosomal regions of the head. Excess residual cytoplasm is stained pink or red. The midpiece shows some red staining, and the tail is stained blue or reddish. Further, it is possible to observe the details necessary for the morphological classification; therefore, we used the modified Papanicolaou staining method for the observation of sperm morphology. Further, we assessed the presence of vacuoles in the sperm head according to the normal morphology rate. However, contrary to expectations, the proportion of these vacuoles increased with an increase in the rate of normal sperm morphology (group A vs. groups B and C, p<0.05). In the present result, the percentage of vacuoles in group A was lower than that in groups B and C, and although the exact cause of the increase in the proportion of sperm-head vacuoles in the normal sperm could not be identified, we should consider the probability that morphologically normal sperm possess vacuoles in the head. Meanwhile, there was no statistically significant difference observed in the proportion of sperm-head vacuoles across any of the age groups.
Further investigations seem to be necessary to compare our observations and the sperm nucleus maturity evaluated with the aniline blue-eosin staining method [36], and to further define the relationship between the sperm-head vacuoles and the ART outcomes.
In conclusion, a majority of the sperm-head vacuoles did not have a statistically significant difference in the case of >4% of normal sperm morphology and depending on the age factor in the routine semen analysis. We should consider the probability of the proportion of sperm-head vacuoles not increasing with age but increasing with the percentage of abnormal sperm morphology. A further study needs to clarify the effects of these vacuoles on the semen parameters and identify an optimal predictive indicator of ART outcomes.
Acknowledgments
The authors would like to express their sincere gratitude to the staff of the Laboratory of Reproductive Medicine.
Notes
No potential conflict of interest relevant to this article was reported.