 |
 |
- Search
| Clin Exp Reprod Med > Volume 41(3); 2014 > Article |
Abstract
Objective
The aim of the present study was to examine whether interactions between polymorphisms in the thyroglobulin and ADAM metallopeptidase with thrombospondin type 1 motif, 16 (ADAMTS16) genes are associated with the development of premature ovarian failure (POF).
Methods
A total of 75 patients with POF and 196 controls were involved in this study. We used a GoldenGate assay to genotype single nucleotide polymorphisms (SNPs). Logistic regression analysis was performed to identify POF-associated polymorphisms and synergistic interactions between polymorphisms in the thyroglobulin and ADAMTS16 genes.
Results
Single gene analyses using logistic regression analysis showed no significant association between polymorphisms in the two genes and POF. In the results from interaction analyses, we found seven synergistic interactions between the polymorphisms in thyroglobulin and ADAMTS16, although there was no combination showing p-values lower than the significant threshold using the Bonferroni correction. When the AG genotype was present at the rs853326 missense SNP, the A and G alleles at the tagging SNPs rs16875268 and rs13168665 showed significant interactions (odds ratios=5.318 and 16.2 respectively; 95% confidence intervals, 1.64-17.28 and 2.08-126.4; p=0.0054 and 0.0079).
Premature ovarian failure (POF) is a heterogeneous and polygenic disease and is characterized by the cessation of the menstrual cycle before the age of forty [1]. Women with POF have high levels of serum FSH (>40 IU/L) and low levels of estradiol. POF may be caused by a decreased number of ovarian follicles during the development of the gonads or by the early loss of follicles, which results from accelerated atresia. Various etiologies for POF have been reported, including autoimmunity, infections, iatrogenic, metabolic, and genetic factors; however, a large portion of cases are still idiopathic [2].
We have previously identified the thyroglobulin (TG) gene as a promising candidate for contributing to POF. Thyroglobulin is the precursor protein for thyroid hormone synthesis and shows a significant association with POF through a single-gene analysis and an epistasis analysis with the hydroxysteroid (17-beta) dehydrogenase 4 gene [3,4]. Thyroid hormones have been reported to stimulate the synthesis of estradiol, progesterone, and androstenedione, which are induced by FSH or LH in mammals, including humans [5,6,7,8]. Defects in the TG gene have been related to the development of thyroid diseases such as thyroid cancer and hypothyroidism [9].
ADAM metallopeptidase with thrombospondin type 1 motif, 16 (ADAMTS16) expresses in granulosa cells in the ovary. A high level of ╬▒2-macroglobulin, which is a substrate of ADAMTS16, was detected in follicular fluid [10], and ╬▒2-macroglobulin has been shown to be involved in the regulation of estradiol production [11]. In addition, we found a significant epistasis effect between the thyroid stimulating hormone beta gene and ADAMTS16 in our previous study [12]. Therefore, we suggested that the ADAMTS16 gene may interact with genes involved in thyroid hormone metabolism, although their interaction had not been previously described. In this study, we hypothesized that the TG gene may interact with the ADAMTS16 gene since both TG and ADAMTS16 have been involved in the regulation of estradiol levels, and therefore examined whether interactions between single nucleotide polymorphisms (SNPs) in TG and ADAMTS16 are associated with POF.
Seventy-five POF patients and 196 female controls were involved in the present study. All of the patients were diagnosed with POF at CHA Hospital (Seoul), according to the following criteria: 1) age less than 40 years, 2) serum FSH levels higher than 40 IU/L, and 3) amenorrhea for >6 months. Sixty-five controls recruited from CHA Hospitals (Seoul and Seongnam) had regular menstrual cycles and had experienced at least one naturally conceived pregnancy. The remaining 131 controls, who were recruited from the city of Chungju as part of a previous study [13], had experienced natural menopause between the ages of 45 years and 60 years. The sample size in the present study was determined to have a statistical power of more than 80%. This study was approved by the Institutional Review Board of CHA University (IRB 2005-003, 10443308-201310-BR-002-01).
The isolation of genomic DNA for all peripheral blood samples was performed using the high-salt buffer method. We diluted all samples to 50 ng/┬ĄL using a 1├ŚTris ethylenediaminetetraacetic acid buffer (pH 8.0) and stored them at -80Ōäā until genotyping. In order to genotype SNPs, we performed a GoldenGate assay (Illumina Inc., San Diego, CA, USA) according to the manufacturer's protocol. This assay employs an allele-specific primer extension method and uses a two color fluorescent labeling system. Fluorescent signals were analyzed by using BeadArray Reader and BeadXpress Reader (Illumina Inc.), and GenomeStudio software (Illumina Inc.) was used for genotype determination.
We performed chi-squared tests for deviations from Hardy-Weinberg equilibrium (HWE). After excluding samples with a call rate less than 80%, logistic regression analysis in an additive model was performed to identify POF-associated SNPs using PLINK software (ver. 1.07, http://pngu.mgh.harvard.edu/~purcell/plink/). In order to identify synergistic interactions between SNPs in the TG and ADAMTS16 genes, logistic regression analyses were also performed using both PLINK and Python programs we coded (version 2.7.3, Python Software Foundation, Wolfeboro Falls, NH, USA). The following analytical method was used. First, samples carrying five genotype patterns (AA, AB, BB, [AA or AB], [AB or BB]) were extracted from all SNPs. Then, we performed logistic regression analyses for each SNP in combination with samples carrying each genotype pattern in another gene. Only combinations with lower p-values than those of single SNPs were included in the results, since these combinations were considered to show synergistic interactions.
The majority of selected SNPs were tagging SNPs and SNPs located in coding regions. Tagging SNPs were identified using a large cohort dataset merged with imputed data using the IMPUTE program obtained from the Korean Genome Epidemiology Study. SNPs located in coding regions, which had minor allele frequencies of 0.01 in Chinese and Japanese subjects, were selected from the National Center for Biotechnology Information SNP database (http://www.ncbi.nlm.nih.gov/projects/SNP/). All SNPs were in HWE. Single SNP analyses using logistic regression analysis in an additive model showed no significant association between SNPs in TG and ADAMTS16 genes and POF (Table 1).
Interaction analyses showed seven significant interactions between the SNPs in TG and ADAMTS16 (Table 2), although there was no combination showing p-values lower than the significant threshold using the Bonferroni correction. When the AG genotype was present at the rs853326 missense SNP, the A and G alleles at the rs16875268 and rs13168665 tagging SNPs showed significant synergistic effects in a dominant model (odds ratios [OR]=5.318 and 16.2; 95% confidence intervals [CI], 1.64-17.28 and 2.08-126.4; p=0.0054 and 0.0079). In addition, when the AC and AG genotypes were present at missense and synonymous SNPs (rs180223, rs2069550), the A and G alleles at rs16875268 and rs13168665 showed significant synergistic effects in a dominant model (OR=5.162 and 15.84; 95% CI, 1.59-16.75 and 2.03-123.5; p=0.0063 and 0.0084). We also found one combination that showed an odds ratio lower than 1. When the GG genotype at rs2069561 was present, the G allele at the missense SNP rs1863968 showed significant synergistic effects in a dominant model (OR= 0.22; 95% CI, 0.07-0.68; p=0.0087).
In the present study, we identified seven synergistic interactions between missense or synonymous SNPs in TG and missense or tagging SNPs in ADAMTS16 although there was no combination showing p-values lower than the significant threshold using the Bonferroni correction.
A large glycoprotein, TG, which is a precursor protein for thyroid hormone synthesis, is secreted into the thyroid follicular lumen. TG is essential for storage of the inactive forms of T3 and T4 in the lumen of thyroid follicles [14]. Mutations in the TG gene have been reported to be associated with various thyroid disorders including hypothyroidism, autoimmune thyroid disease and thyroid cancer [9]. Thyroid hormones have been detected in human follicular fluid, and their receptors have been shown to be expressed in human granulosa cells [15,16,17,18]. It has been also reported that thyroid hormones stimulate the secretion of estrogen and progesterone induced by FSH or LH in mammalian [5,6,7,8]. Therefore, TG may play a crucial role in ovarian function.
Mice carrying mutations in the TG gene have defects in TG folding that result in thyroid endoplasmic reticulum storage disease, which causes deficiencies in TG export [19]. Mutations in the coding regions of TG have caused defective transport of TG in humans [20,21]. These defects arise from endoplasmic reticulum quality control that degrades misfolded or unassembled proteins via a nonlysosomal proteolytic pathway [22,23,24]. Eventually, TG is accumulated in the endoplasmic reticulum, and subsequently thyroid hormone synthesis is reduced [25]. Therefore, the integrity of TG structure is important for the normal synthesis of thyroid hormones. In this study, we found three missense SNPs in TG that participated in synergistic interactions with SNPs in ADAMTS16. We now speculate that the three missense SNPs in TG may affect the integrity of its protein structure and could cause reduced synthesis of thyroid hormones resulting in low levels of estrogen and progesterone.
ADAMTS family members have been shown to be involved in various cellular processes including angiogenesis, development, and ovulation. These proteinases have been associated with many kinds of diseases such as atherosclerosis, asthma and cancer progression [26]. It has been also reported that ADAMTS proteinases play a role in follicle development, ovulation and corpus luteum formation [27,28,29]. ADAMTS16 is expressed in fetal lung and kidney cells and adult brain and ovary cells [30], and it is highly expressed in human granulosa cells in preovulatory follicles [10]. It has also been demonstrated that the expression of ADAMTS16 is induced by FSH and forskolin [10]. A substrate of, ╬▒2-macroglobulin, which is present in higher levels in follicular fluid, has been shown to be involved in the production of estradiol by granulosa cells [10,11]. Therefore, we speculate that the rs1863968 missense SNP within ADAMTS16 or other nonsynonymous SNPs which are in strong linkage disequilibrium with the tagging intronic SNPs may affect the structure of ADAMTS16, thereby altering the efficiency of the cleavage of ╬▒2-macroglobulin and possibly then leading to abnormal regulation of estradiol levels in the ovary.
We investigated whether the structures coded for by TG and ADAMTS16 would be changed by the missense SNPs by using a protein secondary structure prediction program, Jpred3 (http://www.compbio.dundee.ac.uk/www-jpred/) [31,32]. We found differences ranging from 4.2% to 8.4% between the secondary structures of the wild types and the mutant types (data not shown). A striking result was that changes occurred not only at the SNP location but also at regions far from the SNP site. Therefore, we think that structural changes might also occur in regions that we did not examine.
In the present study, we found not only significant associations with an increased risk of POF but also an association with protective effects against POF development. Although the statistical power for this result was lower than 80% (73%), it showed a low odds ratio and a low p-value (OR=0.22; 95% CI, 0.07-0.68; p=0.0087). 85% of controls carried both rs2069561 GG and rs1863968 GG+GA genotypes, but only 55% of POF patients carried those genotypes. Our result suggests that if carriers of the rs2069561 GG phenotype also have the rs1863968 GG+GA genotypes, they have a lower risk of POF development.
In conclusion, synergistic interactions between SNPs located in coding regions or tagging SNPs in TG and ADAMTS16 were found to be associated with POF. Since both TG and ADAMTS16 are believed to play a role in the regulation of estradiol levels in the ovary, we now suggest that synergistic interactions between these polymorphisms or other SNPs tagged by them might lead to inappropriate levels of estradiol in the ovary. Although there was no result that presented lower p-values than the significant threshold using the Bonferroni correction, our results showed p-values<0.01 that could be considered significant. The major limitation of this study is its small sample size. However, compared with other genetic association studies for POF and in light of the low prevalence of POF (1%), our sample size may be considered reasonable. In fact, most of our significant results in this study showed a statistical power greater than 80%. Replication studies with larger sample sizes, which can increase statistical power and can produce lower p-values, are required to confirm our results.
Acknowledgments
The authors thank Sook-Hwan Lee and Sue Kyung Park for offering samples, and we also thank Hwa Beom Shin and Ji Hye Kim for contributing to sample preparation.
References
3. Pyun JA, Kang H, Kim J, Cha DH, Kwack K. Thyroglobulin gene is associated with premature ovarian failure. Fertil Steril 2011;95:397-400.PMID: 20864102.


4. Pyun JA, Kim S, Cha DH, Ko JJ, Kwack K. Epistasis between the HSD17B4 and TG polymorphisms is associated with premature ovarian failure. Fertil Steril 2012;97:968-973.PMID: 22265031.


5. Maruo T, Hayashi M, Matsuo H, Yamamoto T, Okada H, Mochizuki M. The role of thyroid hormone as a biological amplifier of the actions of follicle-stimulating hormone in the functional differentiation of cultured porcine granulosa cells. Endocrinology 1987;121:1233-1241.PMID: 3115761.


6. Wakim AN, Polizotto SL, Burholt DR. Influence of thyroxine on human granulosa cell steroidogenesis in vitro. J Assist Reprod Genet 1995;12:274-277.PMID: 7580025.


7. Wakim AN, Polizotto SL, Burholt DR. Augmentation by thyroxine of human granulosa cell gonadotrophin-induced steroidogenesis. Hum Reprod 1995;10:2845-2848.PMID: 8747030.


8. Spicer LJ, Alonso J, Chamberlain CS. Effects of thyroid hormones on bovine granulosa and thecal cell function in vitro: dependence on insulin and gonadotropins. J Dairy Sci 2001;84:1069-1076.PMID: 11384033.


9. Rubio IG, Medeiros-Neto G. Mutations of the thyroglobulin gene and its relevance to thyroid disorders. Curr Opin Endocrinol Diabetes Obes 2009;16:373-378.PMID: 19633549.


10. Gao S, De Geyter C, Kossowska K, Zhang H. FSH stimulates the expression of the ADAMTS-16 protease in mature human ovarian follicles. Mol Hum Reprod 2007;13:465-471.PMID: 17519324.


11. Ireland JL, Jimenez-Krassel F, Winn ME, Burns DS, Ireland JJ. Evidence for autocrine or paracrine roles of alpha2-macroglobulin in regulation of estradiol production by granulosa cells and development of dominant follicles. Endocrinology 2004;145:2784-2794.PMID: 15001551.


12. Pyun JA, Kim S, Cha DH, Kwack K. Epistasis between polymorphisms in TSHB and ADAMTS16 is associated with premature ovarian failure. Menopause 2014;21:890-895.PMID: 24366283.


13. Chang SH, Kim CS, Lee KS, Kim H, Yim SV, Lim YJ, et al. Premenopausal factors influencing premature ovarian failure and early menopause. Maturitas 2007;58:19-30.PMID: 17531410.


14. Patrick L. Thyroid disruption: mechanism and clinical implications in human health. Altern Med Rev 2009;14:326-346.PMID: 20030460.

15. Wakim AN, Polizotto SL, Buffo MJ, Marrero MA, Burholt DR. Thyroid hormones in human follicular fluid and thyroid hormone receptors in human granulosa cells. Fertil Steril 1993;59:1187-1190.PMID: 8495763.


16. Wakim AN, Polizotto SL, Burholt DR. Alpha-1 and beta-1 thyroid hormone receptors on human granulosa cells. Recent Prog Horm Res 1994;49:377-381.PMID: 8146435.

17. Wakim AN, Paljug WR, Jasnosz KM, Alhakim N, Brown AB, Burholt DR. Thyroid hormone receptor messenger ribonucleic acid in human granulosa and ovarian stromal cells. Fertil Steril 1994;62:531-534.PMID: 8062948.


18. Goldman S, Dirnfeld M, Abramovici H, Kraiem Z. Triiodothyronine (T3) modulates hCG-regulated progesterone secretion, cAMP accumulation and DNA content in cultured human luteinized granulosa cells. Mol Cell Endocrinol 1993;96:125-131.PMID: 8276127.


19. Kim PS, Kwon OY, Arvan P. An endoplasmic reticulum storage disease causing congenital goiter with hypothyroidism. J Cell Biol 1996;133:517-527.PMID: 8636228.



20. Medeiros-Neto G, Kim PS, Yoo SE, Vono J, Targovnik HM, Camargo R, et al. Congenital hypothyroid goiter with deficient thyroglobulin. Identification of an endoplasmic reticulum storage disease with induction of molecular chaperones. J Clin Invest 1996;98:2838-2844.PMID: 8981932.



21. Hishinuma A, Takamatsu J, Ohyama Y, Yokozawa T, Kanno Y, Kuma K, et al. Two novel cysteine substitutions (C1263R and C1995S) of thyroglobulin cause a defect in intracellular transport of thyroglobulin in patients with congenital goiter and the variant type of adenomatous goiter. J Clin Endocrinol Metab 1999;84:1438-1444.PMID: 10199792.


22. Hammond C, Helenius A. Quality control in the secretory pathway. Curr Opin Cell Biol 1995;7:523-529.PMID: 7495572.


23. Kim PS, Arvan P. Folding and assembly of newly synthesized thyroglobulin occurs in a pre-Golgi compartment. J Biol Chem 1991;266:12412-12418.PMID: 2061316.


24. Bonifacino JS, Lippincott-Schwartz J. Degradation of proteins within the endoplasmic reticulum. Curr Opin Cell Biol 1991;3:592-600.PMID: 1772654.


25. Knobel M, Medeiros-Neto G. An outline of inherited disorders of the thyroid hormone generating system. Thyroid 2003;13:771-801.PMID: 14558921.


26. Brocker CN, Vasiliou V, Nebert DW. Evolutionary divergence and functions of the ADAM and ADAMTS gene families. Hum Genomics 2009;4:43-55.PMID: 19951893.



27. Richards JS, Hernandez-Gonzalez I, Gonzalez-Robayna I, Teuling E, Lo Y, Boerboom D, et al. Regulated expression of ADAMTS family members in follicles and cumulus oocyte complexes: evidence for specific and redundant patterns during ovulation. Biol Reprod 2005;72:1241-1255.PMID: 15659705.


28. Espey LL, Yoshioka S, Russell DL, Robker RL, Fujii S, Richards JS. Ovarian expression of a disintegrin and metalloproteinase with thrombospondin motifs during ovulation in the gonadotropinprimed immature rat. Biol Reprod 2000;62:1090-1095.PMID: 10727282.


29. Shindo T, Kurihara H, Kuno K, Yokoyama H, Wada T, Kurihara Y, et al. ADAMTS-1: a metalloproteinase-disintegrin essential for normal growth, fertility, and organ morphology and function. J Clin Invest 2000;105:1345-1352.PMID: 10811842.



30. Cal S, Obaya AJ, Llamazares M, Garabaya C, Quesada V, Lopez-Otin C. Cloning, expression analysis, and structural characterization of seven novel human ADAMTSs, a family of metalloproteinases with disintegrin and thrombospondin-1 domains. Gene 2002;283:49-62.PMID: 11867212.


31. Cuff JA, Clamp ME, Siddiqui AS, Finlay M, Barton GJ. JPred: a consensus secondary structure prediction server. Bioinformatics 1998;14:892-893.PMID: 9927721.


32. Cole C, Barber JD, Barton GJ. The Jpred 3 secondary structure prediction server. Nucleic Acids Res 2008;36:W197-W201.PMID: 18463136.



Table┬Ā2
Synergistic interactions between SNPs within TG and ADAMTS16 in POF
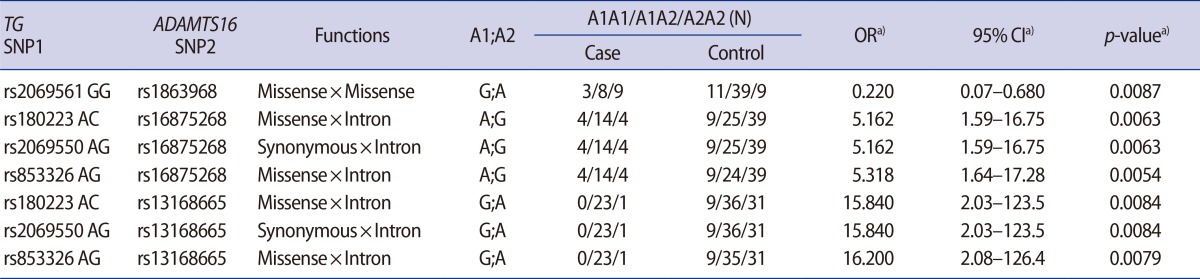
SNP, single nucleotide polymorphism; TG, thyroglobulin; POF, premature ovarian failure; A1, minor allele for SNP2; A2, major allele for SNP2; OR, odds ratio; CI, confidence interval.
a)Values were calculated by logistic regression analyses in a dominant model for the minor allele of SNP2, in combination with the pattern of the genotype at SNP1.







