Association between serum anti-Müllerian hormone level and ovarian response to mild stimulation in normoovulatory women and anovulatory women with polycystic ovary syndrome
Article information
Abstract
Objective
To evaluate the correlation between serum levels of anti-Müllerian hormone (AMH) and ovarian response to mild stimulation in normoovulatory women and anovulatory women with polycystic ovary syndrome (PCOS).
Methods
Seventy-four cycles of mild stimulation (clomiphene citrate+gonadotropin followed by timed intercourse or intrauterine insemination) performed in normoovulatory women (57 cycles) and anovulatory women with PCOS (17 cycles). Ovarian sensitivity was defined by the number of mature follicles (≥14 mm) on triggering day per 100 IU of gonadotropin. A correlation between ovarian sensitivity and the baseline serum AMH level (absolute or multiples of the median [MoM] value for each corresponding age) was calculated. Correlation between ovarian response and serum AMH level was evaluated.
Results
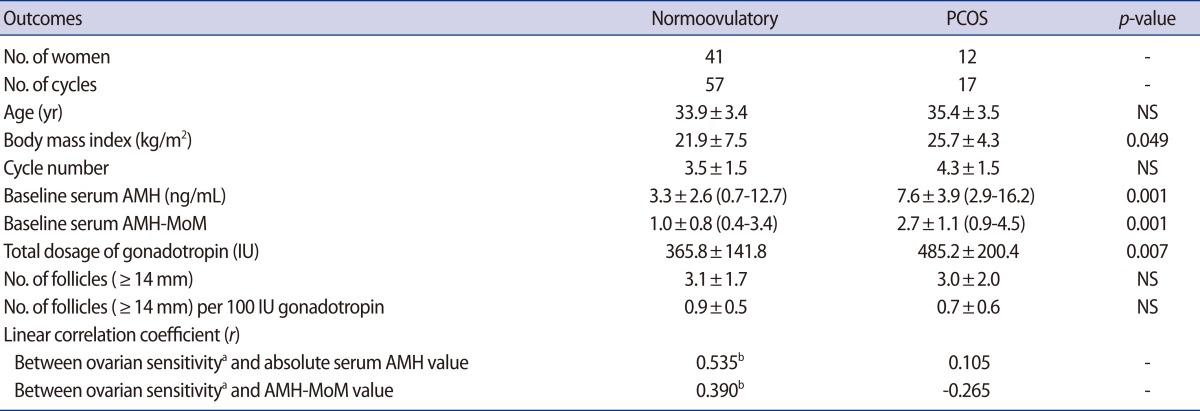
Ovarian sensitivity to mild stimulation was positively correlated with absolute serum AMH (r=0.535, p<0.001) or AMH-MoM value (r=0.390, p=0.003) in normoovulatory women, but this correlation was not observed in anovulatory women with PCOS (r=0.105, p>0.05, r=-0.265, p>0.05, respectively).
Conclusion
Ovarian response to mild stimulation is possibly predicted by the serum AMH level in normoovulatory women, but not in anovulatory women with PCOS.
Introduction
The serum concentration of anti-Müllerian hormone (AMH) has been reported to have a linear relationship with the number of retrieved oocytes in conventional IVF cycles [1-3]. A higher serum AMH level is associated with more retrieved oocytes; in contrast, a lower serum AMH level can predict poor response to ovarian stimulation [4,5].
Interestingly, serum AMH is two to three times higher in women with polycystic ovary syndrome (PCOS) than in women with normal ovaries [6-8]. Many antral follicles exist in women with PCOS, and this may contribute to the high level of serum AMH. In addition, increased AMH production by granulosa cells has also been reported in women with PCOS [9-11]. A serum level of AMH >5 ng/mL was suggested to be the most sensitive and specific diagnostic marker for PCOS [12]. Additionally, serum levels of AMH in patients with ovarian hyperstimulation syndrome (OHSS) are two to three times higher than those seen in normal responders. Therefore, the serum AMH level could potentially serve as a useful marker for identifying high risk patients for OHSS, even in women without PCOS [13].
In non-IVF cycles, ovulation induction (OI) by clomiphene citrate (CC) or FSH and mild ovarian stimulation by CC and/or gonadotropins are commonly used in conjunction with timed coitus or IUI.
In a previous report about the response to CC in women with PCOS, the serum AMH concentration was found to be significantly higher in women who failed to ovulate compared to those who ovulated [14]. This suggests that anovulatory women with PCOS with a relatively high level of serum AMH seem to be resistant to CC and thus need a much higher starting dose of CC. Amer et al. also assessed the ovarian response to OI using a chronic low-dose step up protocol by exogenous FSH in 20 women with CC-resistant PCOS [15]. They found that cycles with a poor response had a significantly higher serum AMH (6.5 ng/mL) compared with those with a good response (4.0 ng/mL). Using a cut-off level of 4.7 ng/mL, the AMH had a sensitivity of 100% and specificity of 58% in predicting poor ovarian response. In women with serum AMH ≥4.7 ng/mL, the duration of FSH stimulation was significantly longer and the total dose of utilized FSH was significantly higher. This suggests that anovulatory women with PCOS who have a relatively high level of circulating AMH seem to be resistant to FSH stimulation. This could be due to the desensitizing effect of AMH on the follicular responsiveness to FSH.
In a mouse model, AMH inhibits initiation of primordial follicle growth and thus functions as an inhibitory growth factor during the early stage of folliculogenesis [16]. A previous in vitro study revealed that AMH has an inhibitory effect on mouse follicle growth by decreasing the sensitivity of ovarian follicles to FSH [17]. A recent in vitro study also demonstrated that AMH inhibits several factors affecting FSH sensitivity in human granulosa cells [18].
A linear relationship between the serum AMH level and ovarian response is well-known in fully stimulated IVF cycles in normoovulatory women, but this relationship was not observed in women with PCOS [7]. It has not been reported whether an ovarian response, even in a mild stimulation cycle, still has a different correlation with the AMH level according to the ovulatory status in infertile women. Here, we assessed the relationship between the serum AMH level and ovarian response in mild stimulation cycles (CC+gonadotropin for timed coitus or IUI) in normoovulatory women and anovulatory women with PCOS.
Methods
Seventy-four cycles of 53 infertile women who received CC+gonadotropin followed by timed intercourse or IUI from 2009 to 2012 were selected retrospectively and several patients received the mild stimulation cycle two or three times. The Institutional Review Board of our hospital approved the use of the patients' medical records. The age of women ranged from 30 to 44 years (mean±SD, 35.7±3.3 years). The infertility diagnoses were unexplained (26 patients), tubal (10 patients), mild male factor (3 patients), and endometriosis (2 patients): all of these were normoovulatory women. Twelve women with anovulatory PCOS underwent 17 cycles. The diagnosis of PCOS was made according to the Rotterdam consensus [19]. All of them had oligo- or amenorrhea with normal TSH, prolactin, day 3 FSH and E2 levels, and classic polycystic ovaries on ultrasound, while other etiologies including congenital adrenal hyperplasia, androgen secreting tumor, and Cushing's syndrome were excluded.
The participants were administered 100 mg/d of CC for 5 days followed by gonadotropins daily or alternate days. Gonadotropins included recombinant FSH (Gonal-F, Serono, Geneva, Switzerland) (56 cycles), or recombinant FSH+highly purified hMG (Menopur, Ferring Malmo, Sweden) (18 cycles). The total amounts of gonadotropin were calculated as per IU in each cycle (mean±SD, 393.2±163.7 IU; range, 150-900 IU). GnRH antagonist for pituitary suppression was not used in all of the cycles.
In the present study, the ovarian response was assessed as the number of mature follicles (≥14 mm) on triggering day. Since the ovarian response is dependent on the amount of exogenous gonadotropin [20], we also assessed the number of mature follicles per 100 IU of gonadotropin, and this was considered to 'ovarian sensitivity'. In addition, the women's age would be a confounding factor; thus the absolute AMH values were converted to multiples of the median (MoM) for each corresponding age. The median values in each age were referenced to the age-specific distribution of serum AMH level for 21,226 Korean women [21].
The baseline serum concentration of AMH was measured as early during the follicular phase as possible. All of the serum samples were assayed using a commercial assay kit according to the manufacturer's protocol (Immunotech, Marseille, France). The intra-assay and inter-assay coefficients of variation were 4.6% and 8.0%, respectively, with a sensitivity of 0.098 ng/mL.
SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis, and the results were considered statistically significant at a p-value of <0.05. The correlation between serum AMH level and ovarian response was quantified using Pearson's coefficient and determined by constructing a fitted curve of the data plotted on linear axes. The slopes of the resulting lines were tested for statistically significant difference from zero by obtaining their p-values from the tables of critical values for the corresponding correlation coefficients. A comparison of continuous variables between the two groups was performed by the non-paired Student's t-test.
Results
Female age was similar between the normoovulatory women and anovulatory women with PCOS, but the body mass index was significantly higher in women with PCOS (Table 1). Anovulatory women with PCOS had a significantly higher baseline serum AMH and AMH-MoM levels. They needed a significantly higher total dosage of gonadotropin but exhibited a similar number of mature follicles compared to the normoovulatory women.
There was a significant positive relationship between ovarian sensitivity and the absolute AMH or AMH-MoM value in the normoovulatory women, but this relationship was not observed in anovulatory women with PCOS (Table 1). In the normoovulatory women, an equation could be obtained: ovarian sensitivity=0.240×[AMH-MoM]+0.650 (p<0.01) (Figure 1). In contrast, in anovulatory women with PCOS, the following equation could be obtained, but it was not significant: ovarian sensitivity=-0.152×[AMH-MoM]+1.156 (p=0.306). In anovulatory women with PCOS, ovarian sensitivity was not related to the AMH-MoM value and even tended to decrease with an increasing AMH-MoM level.

Linear relationships between the baseline serum AMH-MoM level and ovarian sensitivity (number of mature follicles [≥14 mm] per 100 IU of gonadotropin) in mild stimulation in normoovulatory women (upper panel, 57 cycles, p=0.003) and anovulatory women with PCOS (lower panel, 17 cycles, p>0.05). AMH, anti-Müllerian hormone; MoM, multiples of the median; PCOS, polycystic ovary syndrome.
Discussion
In the present study, the ovarian response to mild stimulation by CC+gonadotropin was positively correlated with the serum AMH level in normoovulatory women as it would be in conventional ovarian stimulation in IVF patients. However, the serum AMH level was not related to the ovarian response in anovulatory women with PCOS. The absence of a correlation was also previously reported in conventional ovarian stimulation [7]. The absence of correlation in women with PCOS was also previously reported in conventional ovarian stimulation. Many preantral and small antral follicles exist in women with PCOS; thus the serum AMH concentration is unusually high [7,22-24]. Whilst AMH production from each follicle is high, their levels of production may vary widely. In addition, the threshold of each follicle's ability to respond to ovarian stimulation may be very heterogeneous in women with PCOS; thus an unusually high serum AMH concentration may not be correlated with ovarian response.
When assessing the serum AMH level and response to ovarian stimulation in anovulatory women with PCOS, an unusually high BMI could act as a confounding factor. A high BMI could be correlated with a low serum AMH level as well as low ovarian response [25]. However, a recent report indicates that BMI is not an independent predictor for ovarian response [20]. Moreover, in the present study, neither significant correlations between BMI and AMH nor between BMI and ovarian sensitivity were observed in normoovulatory women (p=0.08 and p=0.16, respectively) or even in women with PCOS (p=0.39 and p=0.23, respectively).
In our study, ovarian sensitivity even tended to be decreased as the serum AMH level increased in women with PCOS. The mechanisms of this phenomenon could be proposed as they have been in other previous reports: the relatively high level of circulating AMH seems to be resistant to CC or chronic low-dose FSH in anovulatory women with PCOS [14,15]. In addition, mild stimulation in women with PCOS might not be sufficient to overcome the negative effect of AMH on follicle growth.
In normoovulatory women, the linear relationship between basal serum AMH level and ovarian response in fully stimulated IVF cycles has been well documented in a previous report from our center [3]. In a fully stimulated IVF cycle, a higher dose of gonadotropin is usually administered to obtain over eight oocytes, which thus appears to overcome the inhibitory effect of AMH on follicle growth. During follicular maturation induced by exogenous gonadotropin, serum AMH levels declined progressively; this reflects the dramatic reduction in the number of small antral follicles due to ovarian stimulation [26]. Reduction of AMH production from the ovary appears to increase FSH sensitivity to larger growing follicles in a fully stimulated cycle.
In the case of mild stimulation, it could be hypothesized that a decrease in AMH production occurs less prominently. Catteau-Jonard et al. investigated whether reduction of AMH production occurs in CC-resistant women with PCOS with at least one dominant follicle after low-dose step-up administration of FSH [27]. They found that a decrease in the serum AMH level was concomitant with the appearance of a dominant follicle. Interestingly, a recent paper reported that serum AMH levels are not decreased as much during mild stimulation as during conventional stimulation [28]. They observed that the mean baseline serum AMH was 4.4 ng/mL in conventional stimulation and 5.6 ng/mL in mild stimulation (p>0.05). At the triggering day, the mean serum AMH values were 1.8 and 3.5 ng/mL, respectively (p<0.05).
In the anovulatory women with PCOS in this study, unlike in the normoovulatory women, the basal serum AMH level was an ineffective predictor of the ovarian response after mild stimulation. This suggests that the basal serum AMH level as an ovarian response predictor in mild stimulation should be carefully applied according to the patient's basal ovulatory status. Our study had some limits because it was retrospective one, with a small number of patients included causing low power, and various PCOS phenotypes were included; however, our results suggest that the serum AMH level is not an appropriate predictor of ovarian response after mild stimulation in anovulatory women with PCOS. Further dynamic and prospective studies are necessary to confirm such a role of AMH and follicle growth after mild stimulation cycles.
Notes
This work was accomplished in Seoul National University Bundang Hospital.
This work was supported by grant no. A120043 from the Korea Health Care Technology R&D Project, Ministry of Health and Welfare, Korea.
No potential conflict of interest relevant to this article was reported.