Blood glucose levels, insulin concentrations, and insulin resistance in healthy women and women with premenstrual syndrome: a comparative study
Article information
Abstract
Objective
To compare the blood glucose levels, insulin concentrations, and insulin resistance during the two phases of the menstrual cycle between healthy women and patients with premenstrual syndrome (PMS).
Methods
From January of 2011 to the August of 2012, a descriptive cross-sectional study was performed among students in the School of Medicine of Jahrom University of Medical Sciences. We included 30 students with the most severe symptoms of PMS and 30 age frequency-matched healthy controls. We analyzed the serum concentrations of glucose, insulin, and insulin resistance by using the glucose oxidase method, radioimmunometric assay, and homeostasis model assessment of insulin resistance equation, respectively.
Results
No significant differences between the demographic data of the control and PMS groups were observed. The mean concentrations of glucose of the two study groups were significantly different during the follicular and luteal phases (p=0.011 vs. p<0.0001, respectively). The amounts of homeostasis model assessment of insulin resistance of the two study groups were significantly different in the luteal phase (p=0.0005).
Conclusion
The level of blood glucose and insulin resistance was lower during the two phases of the menstrual cycle of the PMS group than that of the controls.
Introduction
Premenstrual syndrome (PMS) is a set of psychological and physiological symptoms that can occur during the ten days prior to menses and vanish either shortly before or after the start of menstrual flow [1]. Diagnosis of PMS relies on the recognition and report of the symptoms by the patients who experience it because there is no identifying test for detecting PMS. Although the precise pathophysiology of PMS remains unknown, it is suspected that PMS may be the result of dysregulation of the serotonergic system. In fact, serotonergic reuptake inhibitors are usually a successful treatment for severe forms of PMS [2]. On the other hand, some studies have shown that women who experience PMS are more likely to eat sweets and carbohydrates in the luteal phase [3,4]. Studies on animal models have shown that brain serotonin levels were increased by carbohydrate ingestion [5,6]. In addition, serotonin not only regulates glucose and estradiol levels but also affects insulin resistance and blood glucose levels, and it can stimulate or intensify PMS symptoms [7,8]. According to these previous findings and since evidence regarding this issue is inadequate, we hypothesized that the levels of insulin, insulin resistance, and glucose would be different in the follicular and luteal phases of the menstrual cycle in women with PMS compared to controls.
Methods
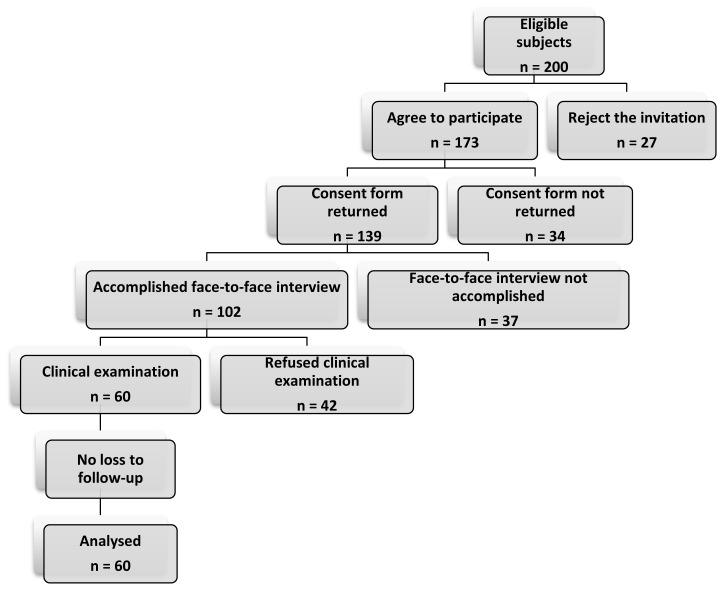
The present study is a descriptive cross-sectional survey that was carried out among students in the School of Medicine of Jahrom University of Medical Sciences between January 2011 and August 2012. Two hundred students were considered for eligibility, and in the end, 60 women aged 18 or above were consecutively enrolled into the study (Figure 1). Of them, the 30 students with the most severe symptoms of PMS, hereafter the "women with PMS," were compared with 30 controls with a normal menstrual cycle. The control subjects were frequency matched to the patients by age. None of the women in the two study groups had a history of diabetes, chronic psychiatric or metabolic disease, thyroid disease, or polycystic ovary syndrome. We excluded those women who were under replacement or hormonal contraception therapy or were currently using selective serotonin reuptake inhibitors, triptan medications, antidepressant medications, or monoamine oxidase inhibitors during the previous two months. Participants who were pregnant, planning to become pregnant, failed to complete the questionnaire, or were unwilling to participate voluntarily were excluded from the study. This study complied with all of the principles of research ethics regulations which have been adopted by the Iranian Ministry of Health. Likewise, the study protocol was approved by the Institutional Review Board (IRB) of Jahrom University of Medical Sciences. Although the IRB approved this study, the medical students of our own institution might be vulnerable subjects. However, ethical approval was obtained from the Ethics Committee and all of the participants gave their written informed consent.
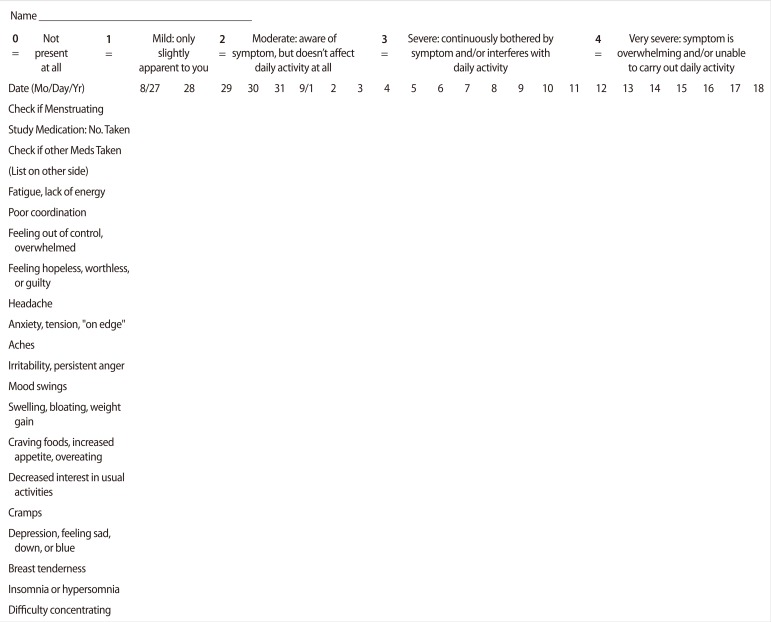
All of the participants were investigated with a screening history and physical examinations by a trained staff person to assess that the subjects were in good general health. Screening included a face-to-face interview and standardized questionnaires including personal data and clinical measurements such as age, drug consumption during the past two months, obesity, and medical or family history of PMS. Two months of prospective symptom charting was used to confirm the PMS status of participants through the Penn Daily Symptom Report (DSR) (Figure 2) as a valid and reliable tool for diagnosis of PMS [9]. Subjects were required for two consecutive cycles to have at least a 30% increase in total symptoms reported in the follicular phase vs. the luteal phase, with a minimum luteal phase score of 80 for confirmation of PMS. Participants were scheduled for admissions to the laboratory clinic of the Jahrom Medical University four times in a month (the 7th, 13th, 21st, and 26th days of their menstrual periods). Each participant functioned as her own control, and all measurements were taken under both cycle phase conditions. The first admission could be either a luteal or follicular phase admission according to the menstrual cycle pattern of the subjects. The onset of menses was considered to be day 1, and the presence of menstrual bleeding was used to determine the follicular phase, so days 7 through 13 after the onset of menses were considered to be the follicular phase. A surge detection kit (Pars Azmoon, Tehran, Iran) for urinary LH was used to determine the luteal phase. Participants were trained to use the LH surge detection kits and told to alert the investigator at the time of the LH peak, so days 21 through 26 after onset of the study (7 to 10 days after the LH peak) were considered to be the luteal phase. Plasma assays of progesterone and estradiol was used to confirm the cycle phase.
Subjects were asked to remain fasted for at least 12 hours and not to have much physical activity before admission to the clinic, then referred to the laboratory at 8:00 AM. Insulin resistance was evaluated through insulin and glucose concentrations by use of the homeostasis model assessment of insulin resistance (HOMA-IR) equation [10]. Blood samples were taken at postprandial 0 and 2 hours and plasma was reserved with ethylenediaminetetraacetic acid, then the serum was separated immediately by centrifugation at 3,000 rpm for a period of 15 minutes. The samples were processed directly or in the 7 to 10 days following preservation at -70℃. Glucose measurements (inter-assay coefficient of variation [CV] 2.6%, intra-assay CV 2.1%) were performed using the glucose oxidase method. Radioimmunometric assay was used to determine the serum concentrations of insulin, estradiol, and progesterone. The inter- and intra-assay coefficients of the variation for insulin were 5.5 and 4.8%, respectively. The inter-assay variation coefficient was 8.97% for progesterone and 9.21% for estradiol, whereas the intra-assay values were 8.03% for progesterone and 8.69% for estradiol.
1. Statistical analysis
Based on a power of 90% to find a significant difference regarding the preliminary study [11] (p=0.05, 2-sided), 19 subjects were required for each study group. We planned to enroll 30 subjects per group to compensate for any refusal to provide data or nonvaluable subjects. Results were reported as mean±SD or median for quantitative variables, and percentages for categorical variables. The continuous variables that failed the normality test were logarithmically transformed before analysis. The variables transformed were insulin, glucose, and HOMA-IR. Statistical differences are accord with log-transformed data analyses, but the means of both transformed and untransformed data are presented in tables. The Student's t-test was used to compare between parametric data sets. A 2-sided p-value of 0.05 or less was considered statistically significant. All of the statistical analyses were performed using SPSS ver. 15.0 (SPSS Inc., Chicago, IL, USA) for Windows.
Results
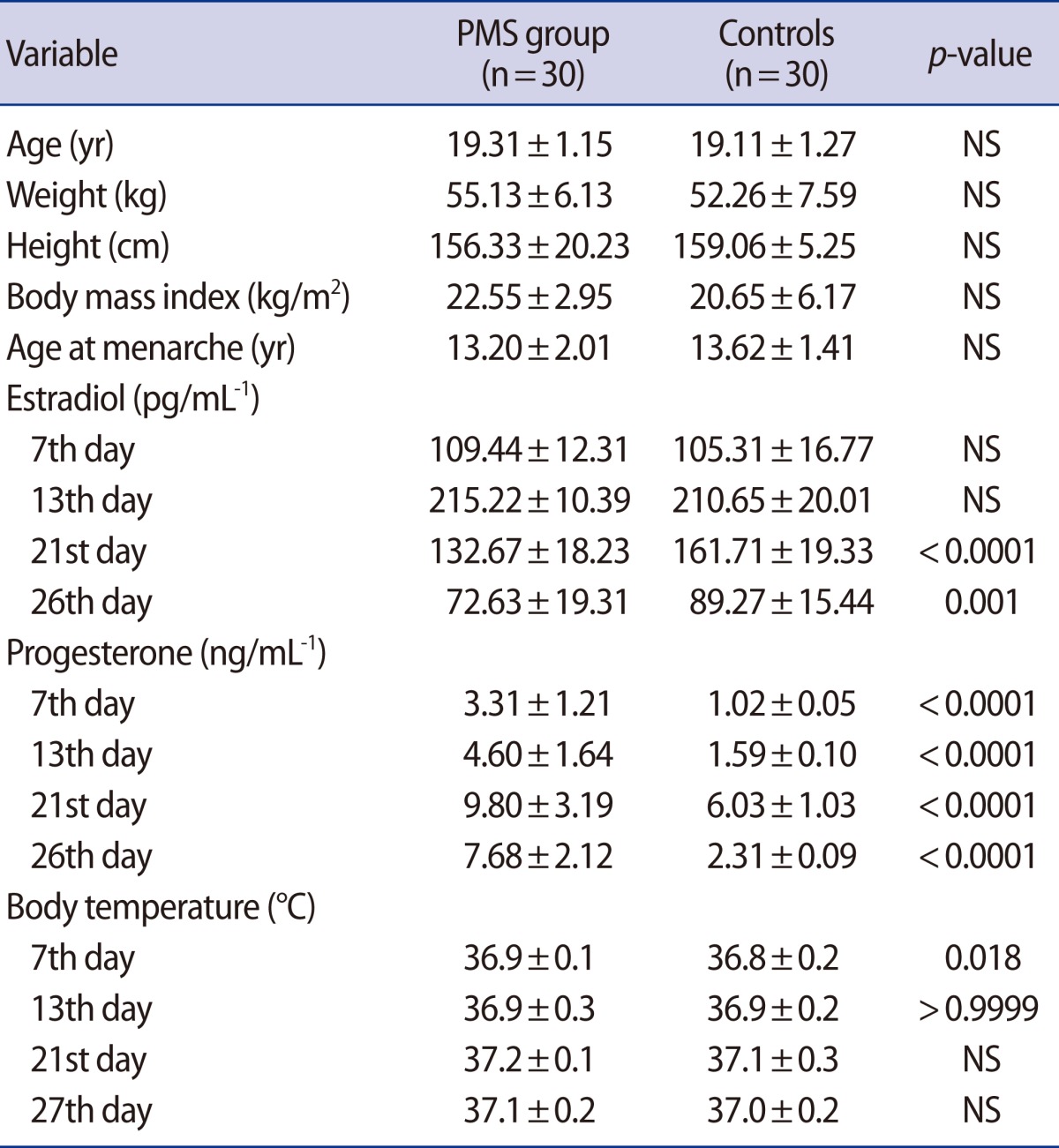
All of the subjects completed the study and none of them were excluded from it. All participants were aged 18 to 22 years. The average age of the participants was 19.21±1.21 years. The mean age at menarche of the participants was 13.41±1.7 years (range, 11 to 16 years). No significant differences between baseline characteristics of patients and control groups were observed. There were significant differences in the estradiol assay of the two groups at days 21 and 26 of menses. However, significant differences in progesterone levels were observed during all of the days of the study period. The clinical data and demographic characteristics of the participants in the two study groups are shown in Table 1.
The mean score of the 7th day of the menstrual cycle on the Penn DSR was 15.5±10.5; the mean score of the 13th day of the menstrual cycle was 30.2±12.5; the mean score of the 21st day of the menstrual cycle was 167.5±33.5; and the mean score of the 27th day of the menstrual cycle was 170.6±29.3 in the PMS group. There were significant differences between the mean scores on the Penn DSR between the cases and controls during the two phases of the menstrual cycle (Table 2).

The mean scores on the Penn DSR during the follicular and luteal phases of the menstrual cycle in cases and controls
The mean concentrations of insulin, glucose, and amounts of HOMA-IR are shown at days 7, 13, 21, and 27 after the onset of menses in the two study groups in Figure 3. There were lower mean concentrations of glucose and amounts of HOMA-IR in the PMS group than in the controls in the two phases of the menstrual cycle. The mean concentrations of glucose of the two study groups were significantly different during the follicular and luteal phases (p=0.011 vs. p<0.0001, respectively). No significant differences between mean concentrations of insulin during the two phases of the menstrual cycle were found between the control and PMS groups (p=0.746 vs. p=0.507, respectively). The amounts of HOMA-IR of the two study groups were significantly different during the luteal phase (p=0.0005), but they were not significantly different during the follicular phase (p=0.368). Table 3 shows the mean concentrations of insulin, glucose, and amounts of HOMA-IR in the two phases of the menstrual cycle of both study groups.
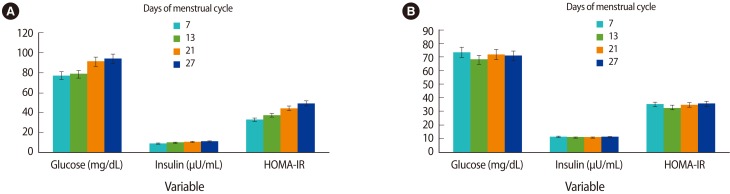
The mean concentrations of insulin, glucose, and amounts of HOMA-IR increase consecutively at the days of 7, 13, 21, and 27 after the onset of menses in controls (A). The mean concentrations of insulin, glucose, and amounts of HOMA-IR differ at the days of 7, 13, 21, and 27 after the onset of menses in the PMS group (B). HOMA-IR, homeostasis model assessment of insulin resistance; PMS, premenstrual syndrome.
Discussion
To the best of our knowledge, this is the first comparative study aimed at investigating the differences in insulin resistance, insulin, and glucose concentrations between women with PMS and women with a normal menstrual cycle. As fluctuations of steroidal hormones have been marked on certain days of the menstrual cycle, we have selected these times, specifically, 7, 13, 21, and 27 days after the onset of menses. Based on the theory of which steroidal hormones affect glucose homeostasis, we had hypothesized that any derangement of steroidal hormone fluctuations would cause further stimulation of PMS symptoms on the specific days of the menstrual cycle mentioned above. According to the results obtained from the present study, the decreased level of blood glucose and insulin resistance in the PMS group during the luteal and follicular phases compared to controls can exacerbate PMS symptoms. Although our findings demonstrated the significantly increased level of blood glucose and insulin resistance in the controls during the luteal phase compared to the follicular phase, reported data from the PMS group showed no significant differences between those variables during the luteal and follicular phases.
The body mass index (BMI) of the subjects with PMS was greater than the BMI of the controls. These data were in consonance with the results found by previous studies [12]. During the present study, we observed that glucose concentrations in the luteal phase were significantly higher than in the follicular phase in controls. Trout et al. [13] found significant differences in the blood glucose levels between the follicular and luteal phases in women with type 1 diabetes. In that study, they concluded that increased blood glucose levels during the luteal phase may be the result of elevated insulin-independent glucose disposal. The elevated glucose during the luteal phase in healthy women due to increased progesterone levels prevents the entry of glucose into the insulin-sensitive tissues, either in combination with estrogen or alone [14]. Some other studies performed in healthy women have documented that blood glucose did not change across different phases of the menstrual cycle [15,16]. On the other hand, others have theorized that the energy consumption during the luteal phase is due to fat instead of carbohydrate metabolism, resulting in the increased glucose levels in the luteal phase. This metabolism pattern is also attributed to the role of estradiol in relation to carbohydrate metabolism and loading in the luteal phase [17,18]. In the present study, the authors found an increase in insulin resistance and the progesterone level in healthy women during the luteal phase of the menstrual cycle in comparison to the follicular phase. This suggests that the reduction in insulin sensitivity during the luteal phase depends on the increased level of progesterone. In the study of Escalante Pulido and Alpizar Salazar [19] a trend was shown towards a correlation between increased plasma progesterone levels and decreased insulin sensitivity.
In our study, the blood glucose levels and insulin resistance were significantly lower during the luteal and follicular phases in the PMS group compared to those of the controls. Decreased blood glucose levels on the 13th day of menses were the lowest among the days of the menstrual period tested. Likewise, during the luteal and follicular phases, no significant differences in the insulin concentration between the two study groups were observed. Although undocumented evidence indicates that the signs and symptoms of PMS occur a few days before the onset of menstruation, this study found that the disturbances of glucose homeostasis began from the follicular phase and continued into the luteal phase. It is known that vitamin D stimulates insulin secretion via its direct action on the pancreatic beta cells and its indirect action by extracellularly normalizing calcium levels [20]. In addition, the positive effects of progesterone on the insulin concentration have been observed in both humans and monkeys after glucose administration [21]. Based on such evidence, it has been suggested that patients with PMS experience insulin reduction leading to a decreased level of progesterone and vitamin D [22].
According to the data of this study, the exact reasons for the lack of significant differences in insulin concentration observed between the two study groups are not entirely clear; recruitment procedures and differences in environmental background and/or other unknown associated factors could have played a role. It is also possible that the unidentified variables that contribute to changes in insulin secretion during the menstrual cycle may be static, that is, either constantly depressed or constantly elevated in patients with PMS. On the other hand, whenever the concentration of blood glucose is low, insulin secretion is reduced [23]. Liver function regulates the rate of glucose output. Therefore, because of liver dysfunction in women with PMS, the rate of glucose is reduced.
Sex steroids affect the synthesizing of nervous neurotransmitters and the serotonergic system, indicating that serotonergic transmission partly influences behavior. Moreover, serotonin terminals could apply a depressing influence on brain areas involving the amygdala, which are under a parallel, freelance activating effect of sex steroids [24,25]. Alternatively, nervous neurotransmitters affect the energy balance, glucose homeostasis, insulin resistance, and extra micronutrient levels [26]. The brain neurotransmitter serotonin and inhibitory amino acid gamma-aminobutyric acid (GABA) have been linked to PMS [7]. A recent study has reported the novel mechanism of serotonin's action in beta cells of the pancreas. Serotonin controls the release of insulin, which is the most important hormone in the regulation of the blood glucose concentration of animals and humans under normal conditions [27]. Hence, the dysregulation of the serotonergic system in patients with PMS indirectly affects blood glucose levels. Reduction of blood glucose levels on the 13th day of the menstrual cycle may be due to the decreased level of progesterone and increased level of estradiol that accelerate glucose uptake into the musculoskeletal system [28].
Previous studies have indicated differences in insulin resistance during the menstrual cycle in nondiabetic women. However, those studies did not screen for the absence or presence of PMS, and it is not entirely clear if absence of this variable would lead to the reported differences in insulin resistance. In the laboratory study of Valdes and Elkind-Hirsch [29], significant differences in the insulin resistance of healthy women during the follicular and luteal phases were observed. In one other study, no significant differences in insulin resistance during the menstrual cycle phases were seen either in healthy women or in patients with PMS [11]. Widom et al. [30] indicated that women who exhibited significantly higher estradiol levels in the luteal phase were observed to have significantly lower insulin resistance during this same phase of the menstrual cycle. In contrast, women who did not show differences in estradiol levels between the follicular and luteal phases of the menstrual cycle exhibited no phase dependent-changes in insulin resistance. As a result, in this particular study the estradiol levels seemed to influence possible menstrual cycle changes in insulin resistance.
The strength of the present study is that it is a comparative study, comparing the blood glucose level, insulin concentration, and insulin resistance on the different days of the menstrual period between healthy women and those with PMS. The limitations of our study include the small number of subjects studied with insufficient power to show significant differences in insulin concentration between the two study groups.
In conclusion, the findings of the present study revealed the lower level of blood glucose and insulin resistance in the PMS group during the follicular and luteal phases of the menstrual cycle compared to those of healthy women. The lower blood glucose on the 13th day after the onset of menses in the PMS group compared to controls was noticeable. Likewise, according to the data from this study, hypoglycemia is a stimulating factor of PMS symptoms.
Notes
Hereby, the authors acknowledge the authorities of Jahrom University of Medical Sciences for financial support.
No potential conflict of interest relevant to this article was reported.