Cessation of gonadotropin-releasing hormone antagonist on triggering day in flexible multiple-dose protocol: A randomized controlled study
Article information
Abstract
Objective
To investigate outcomes of stimulated IVF cycles in which GnRH antagonist was omitted on the ovulation triggering day.
Methods
A total of 86 women who underwent controlled ovarian hyperstimulation with recombinant FSH and GnRH antagonist flexible multiple-dose protocols were recruited and prospectively randomized into the conventional group (group A) or cessation group (group B). The GnRH antagonist, 0.25 mg/day of cetrorelix, was started when the leading follicle reached 14 mm in diameter and was continuously administered until the hCG triggering day (group A, 43 cycles) or until the day before hCG administration (group B, 43 cycles). The maturity of oocytes, fertilization rate, embryo quality, and implantation and clinical pregnancy rates were evaluated.
Results
The duration of ovarian stimulation, total dose of gonadotropins, serum estradiol levels on hCG administration day, and number of oocytes retrieved were not significantly different between the two groups. The total dose of GnRH antagonist was significantly lower in group B than group A (2.5±0.9 vs. 3.2±0.8 ampoules, p<0.05). There was no premature luteinization in any of the subjects. The proportion of mature oocytes and fertilization rate were not significantly different in group B than group A (70.7% vs. 66.7%; 71.1% vs. 66.4%, respectively). There were no significant differences in the implantation or clinical pregnancy rates.
Conclusion
Our prospective randomized study suggested that cessation of GnRH antagonist on the hCG administration day during a flexible multiple-dose protocol could reduce the total dose of GnRH antagonist without compromising its effects on pregnancy rates.
Introduction
The GnRH antagonists have emerged as an alternative for GnRH agonists in preventing a premature LH surge in assisted reproductive technology (ART). They have a distinct advantage over GnRH agonists in that they induce an immediate decrease in circulating gonadotropin levels with rapid reversal [1-3]. However, recent meta-analyses have shown significantly lower pregnancy rates, serum estradiol levels on the hCG day, and number of oocytes retrieved in GnRH antagonist cycles compared with GnRH agonist cycles [4-6].
Possible extrapituitary actions of GnRH antagonist and the role of LH in the follicular phase have again become a matter of debate because GnRH antagonist can completely suppressed serum LH level at a critical stage of follicular development. FSH is known to be a key factor in gonadal differentiation and maturation [7]. It is also responsible for the induction of LH receptors on granulosa cells. In addition, by its synergistic effect with LH, FSH activates the aromatase system, which enables the follicle to produce E2, ovulate, and luteinize in response to the LH surge [8].
There is still no consensus on the optimal GnRH antagonist protocol. Thus, additional efforts are needed to identify the optimal stimulation protocols to achieve better follicular and embryonic development and to improve the pregnancy rates in controlled ovarian hyperstimulation (COH) using GnRH antagonist. Withdrawal of GnRH antagonist can immediately reverse its antagonizing effects; therefore, cessation of GnRH antagonist administration on the hCG day could remove the possible detrimental effect of GnRH antagonist on final oocyte maturation. We previously demonstrated in retrospective study that cessation of GnRH antagonist on the day of hCG administration during a flexible multiple-dose protocol could reduce the total dose of GnRH antagonist and improve oocyte and embryo quality [9].
The aim of this prospective randomized trial is to compare COH outcomes according to whether or not GnRH antagonist is administrated on the hCG day in GnRH antagonist flexible multiple-dose protocols.
Methods
1. Patients
This study was an investigator-initiated trial involving a total of 86 randomized patients, included from June 2007 to August 2009. The inclusion criteria were: 1) 20 to 40 years old, 2) baseline FSH<12 mIU/mL, 3) ≤3 previous IVF attempts, and 4) regular menstrual cycle (24 to 35 days). The couples with severe male factor such as oligoasthenoteratozoospermia or nonobstructive azoospermia with chromosomal abnormality, polycystic ovarian syndrome [10], and other endocrine abnormalities were excluded. This study was approved by the Institutional Review Board of Seoul National University Bundang Hospital (IRB No. B-0710/050-001). Each of the patients had given written authorization at the time of treatment for the future use of their clinical data. In addition, this clinical trial study was registered at ClinicalTrials.gov (NCT00571870).
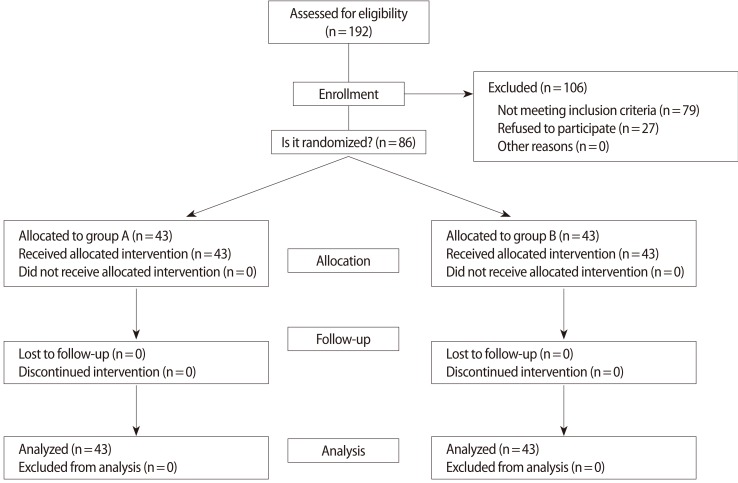
A total of 86 women who underwent COH with recombinant FSH and GnRH antagonist flexible multiple-dose protocols were recruited. The GnRH antagonist, 0.25 mg of cetrorelix, was added when the leading follicle reached a diameter of 14 mm. On stimulation day 1, the patients (n=86) were randomized to either continue GnRH antagonist daily until the day of hCG administration (n=43, group A; GnRH antagonist use group) or to omit the day of hCG administration (n=43, group B; GnRH antagonist omitted group) (Figure 1).

Schematic diagram of controlled ovarian hyperstimulation protocol. On menstrual cycle day 3, recombinant FSH (rFSH) was started and the dose was adjusted individually. Once the largest follicle reached 14 mm in mean diameter, 0.25 mg of GnRH antagonist was started. 10,000 IU of urinary hCG (u-hCG) or 250 µg of recombinant hCG (r-hCG) was administered when the leading follicle reached 18 mm in mean diameter. The GnRH antagonist was administered daily until the day of hCG administration (group A) or the day before hCG administration (group B). MCD, menstrual cycle day.
2. Randomization
Eligible women were recruited and randomly assigned to either group by means of computer-generated random numbers. Selection into the groups and randomization into the appropriate treatment protocol were performed by a coordinating nurse, using a series of consecutively numbered sealed opaque envelopes; therefore, the sequence of allocation was concealed (Figure 2). The study was single-blinded, because the clinicians were not aware of the treatment group, but the patients were aware of the treatment group.
3. Controlled ovarian hyperstimulation protocols
Recombinant FSH (Gonal-F, Serono, Geneva, Switzerland) was started on the second or third menstrual cycle day without previous oral contraceptive pretreatment. The starting dose of rFSH was 150 IU/day in all of the patients ≤35 years of age and 225 IU/day in all of the patients >35 years of age. The rFSH dose was fixed for the first five days, after which the dose could be individualized. The GnRH antagonist, 0.25 mg of cetrorelix acetate (Cetrotide, Serono), was added daily, starting when the leading follicle reached 14 mm in diameter. When the leading follicle reached a mean diameter of 18 mm or two follicles or more reached a diameter of 17 mm, 10,000 IU of urinary hCG (Pregnyl, Organon, the Netherlands) or 250 µg of recombinant hCG (Ovidrel, Serono) were intramuscularly or subcutaneously injected. In group A, the GnRH antagonist continued to be used until the hCG administration day. In group B, the GnRH antagonist was not administered on the hCG day (Figure 1). After hCG injection, oocyte retrieval was performed 35 to 36 hours later.
At the time of oocyte retrieval, the maturity of oocytes was assessed. They were classified as mature (MII), intermediate, or immature oocytes (including germinal vesicles) according to the cumulus/corona morphology, cytoplasmic clarity, zona thickness, and extent of the perivitelline space [11]. Embryonic morphologic development was assessed according to four grades (I-IV) on culture according to the regularity of blastomeres, the percentage of anulceate fragments, and all dysmorphic characteristics of the embryos: 1) grade I: 0% anucleate fragments, regularity of blastomeres, and no apparent morphologic abnormalities; 2) grade II: <20% anucleate fragments, regularity of blastomeres, and no apparent morphologic abnormalities; 3) grade III: 20% to 50% anucleate fragments, irregularity of blastomeres, and no apparent morphologic abnormalities; and 4) grade IV: ≥50% anucleate fragmentation, irregularity of blastomeres, and apparent morphologic abnormalities. A good-quality embryo was defined as those of morphologic grade I or II, with at least four blastomeres on day 2 and at least seven blastomeres on day 3 after fertilization.
4. Fertilization and embryo transfer
After oocyte maturation was achieved, the oocytes were inseminated with fresh pretreated sperm. If fertilization had failed in previous IVF cycles or the cause of infertility was a male factor, ICSI was performed. Fertilization was assessed 16 to 18 hours after insemination or injection, where the presence of two pronuclei was recorded as normal fertilization. Up to four embryos were transferred 2 or 3 days after the oocyte retrieval. Most of the patients underwent transfer of 2 to 3 embryos. In case of old age (>35 years old) with poor embryo development, up to four embryos were transferred. Pregnancy was initially assessed using serum β-hCG 14 days after oocyte retrieval. 50 mg/day of progesterone in oil (Progest, Samil Co., Seoul, Korea) was started intramuscularly from the oocyte retrieval day until pregnancy testing, and was continued until 8 weeks if pregnant. Clinical pregnancy was defined by the presence of an intrauterine gestational sac with visible fetal heartbeats 3 to 4 weeks after embryo transfer.
5. Hormonal measurements
On the day of hCG administration, patients' sera were obtained and serum levels of LH, progesterone, and E2 were determined. Serum levels of LH were measured by immunoradiometric assay using a commercial kit (Biosource, Nivelles, Belgium). The detection limit and intra- and inter-assay coefficients of variation (CVs) were 0.2 mIU/mL, 3.2%, and 6.7%, respectively. Estradiol and progesterone concentrations were measured by RIA using commercial kits (Biosource). The detection limits and the intra- and interassay CVs were 10 pg/mL, 4.9%, and 5.2% for E2, and 0.02 ng/mL, 3.3%, and 7.1% for progesterone, respectively.
A premature LH rise was defined as LH≥10 mIU/mL and premature progesterone rise as progesterone ≥1.0 ng/mL. The combination of the above-mentioned conditions (LH≥10 mIU/mL and progesterone ≥1.0 ng/mL) was indicated as premature luteinization, as described previously [12]. The main outcomes were maturity of oocytes, fertilization rate, embryo quality, implantation rate, pregnancy rate, and amount of GnRH antagonist used.
6. Statistical analysis
The data were analyzed using the Mann-Whitney U test for continuous variables and the chi-squared or Fisher's exact tests for categorical variables. The statistical software package SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA), was used for statistical analysis, and a p<0.05 was considered to be statistically significant.
Results
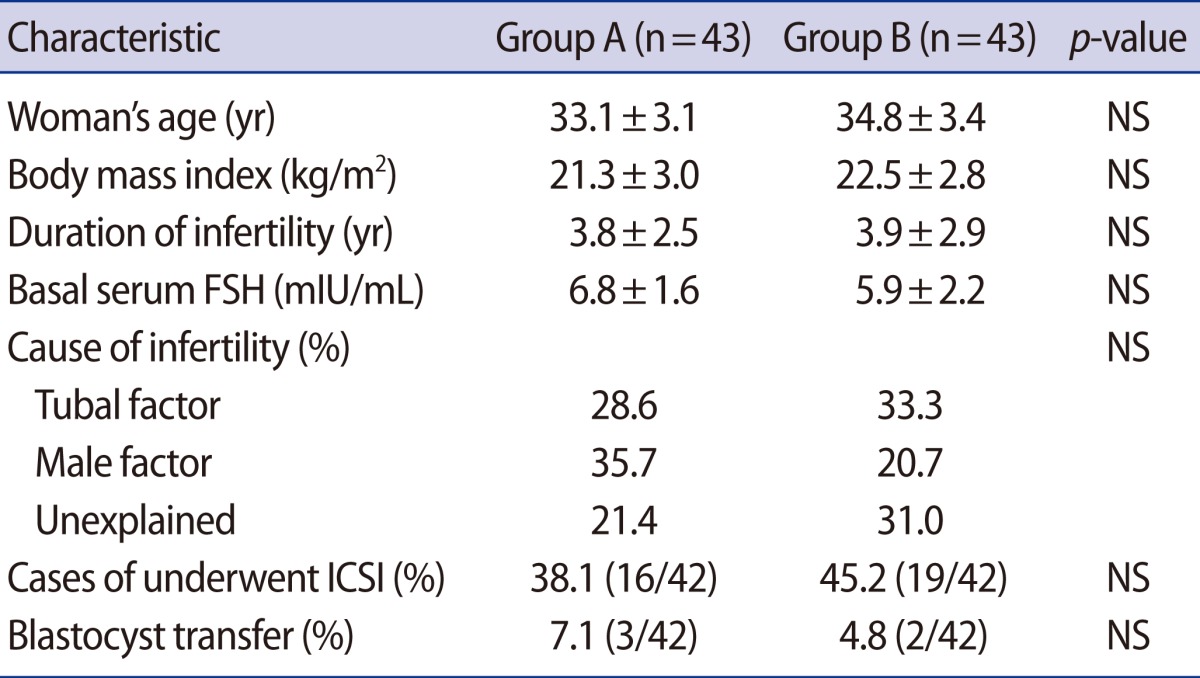
The patients' characteristics are described in Table 1. There were no statistically significant differences in clinical characteristics, such as age, body mass index, duration of infertility, basal serum FSH levels, or distribution of the causes of infertility between the two groups.
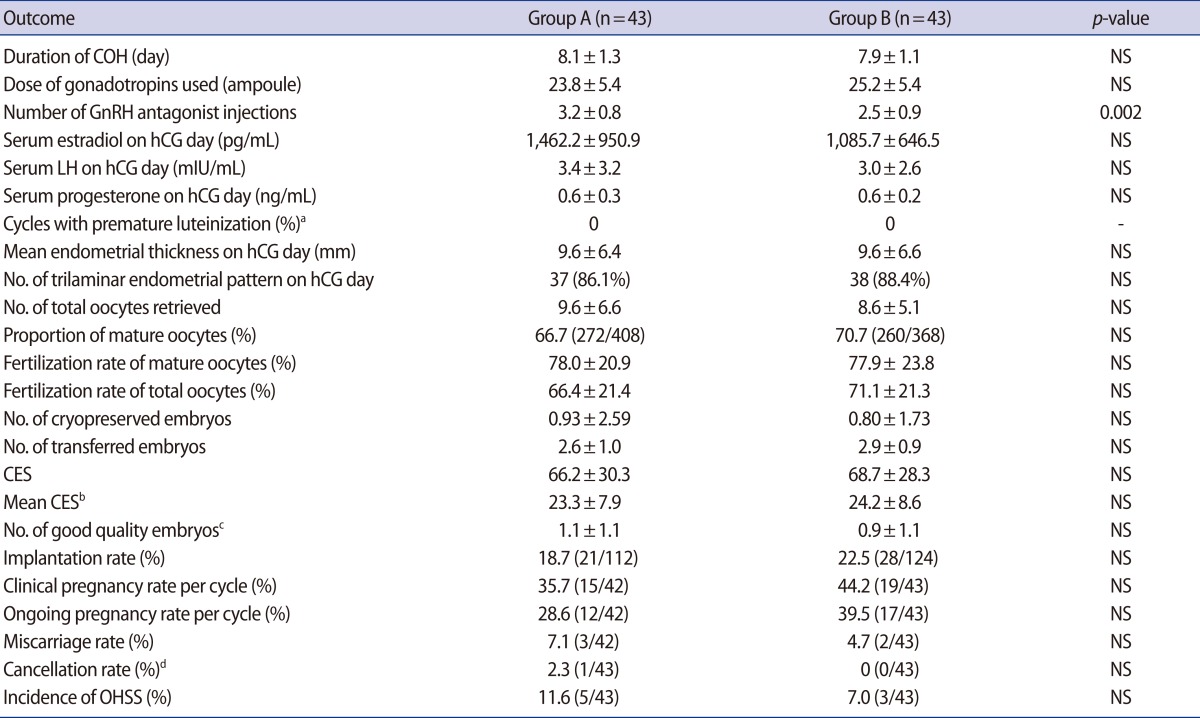
The duration of COH, total dose of gonadotropins, estradiol levels on the hCG day, and number of oocytes retrieved were not significantly different between the two groups. As expected, the total dose of GnRH antagonist was significantly lower in group B compared to group A (2.5±0.9 vs. 3.2±0.8 ampoules, p=0.002). There was no case of premature luteinization. The ratio of mature to total retrieved oocytes and the fertilization rates of mature and total oocytes were comparable for the two groups. The mean endometrial thicknesses and rates of a trilaminar endometrial pattern on the hCG day were 9.6±6.4 mm vs. 9.6±6.6 mm, and 86.1% (37/43) vs. 88.4% (38/43), respectively (group A vs. B, p>0.05). The number of good quality embryos was also comparable for the two groups. There were no significant differences in other outcomes, such as the fertilization rate, clinical pregnancy rate, or implantation rate between the two groups. The implantation and clinical pregnancy rates were higher in group B, but the differences were not statistically significant (Table 2).
Discussion
Many years have passed since GnRH antagonists were introduced to prevent premature LH surges during stimulated cycles. The use of GnRH antagonists has several advantages, such as being more patient-friendly, avoidance of ovarian cyst formation, shortening of ovarian stimulation duration, and a reduction in the incidence of ovarian hyperstimulation syndrome (OHSS) [13]. However, the use of GnRH antagonist in COH remains less popular than GnRH agonist because of trends toward lower pregnancy rates in IVF cycles using GnRH antagonists compared with the GnRH agonist long protocol, as reported in meta-analyses [5,14]. There have been various attempts to modify the GnRH antagonist protocol and to improve COH outcomes. These involve pretreatment with 17β-estradiol [15] the intercycle administration of a GnRH antagonist [16], pretreatment with oral contraceptives [17], or modifications of initiation timing [18,19]. However, a meta-analysis of 27 randomized controlled trials that included recent reports also showed significantly lower clinical and ongoing pregnancy rates in the antagonist group [5]. It remains necessary to determine more optimized stimulation protocols to achieve better follicular and embryonic development and to improve pregnancy rates in COH using a GnRH antagonist.
The role of LH in follicular development has again become a matter of debate, because GnRH antagonists can completely deprive secretion of LH at a critical stage for follicular development. Ovarian follicles have development-related requirements for stimulation by LH; that is, there is a "threshold" for LH requirements during folliculogenesis. However, the high level of LH above the threshold could suppress aromatase activity and inhibit cell growth. These findings have been observed by different investigators and are known as the "LH window" during the follicular phase of menstrual and induced cycles. In view of the decreased probability of pregnancy associated with low LH levels, similar effects were also observed; hMG leads to a significantly higher clinical pregnancy rate than recombinant FSH (rFSH) alone in IVF cycles of normogonadotropic GnRH agonist down-regulated patients [20]. Moreover, in many studies, GnRH antagonist protocols were associated with significantly lower serum E2 levels on the hCG administration day and a significantly lower number of retrieved oocytes [21-23] than were GnRH agonist protocols.
We previously demonstrated that the group in which GnRH antagonist was omitted on the day of hCG administration had comparable COH outcomes while reducing the total doses of GnRH antagonist during a flexible multiple dose protocol in a retrospective study [9]. In a previous retrospective study, cessation of GnRH antagonist on the day of hCG administration could have improved the quality of retrieved oocytes. Although the total number of retrieved oocytes was not significantly different in between the two groups, the oocyte maturity and fertilization rate were significantly higher in the cessation group without premature luteinization occurring. These results suggest that the cessation of GnRH antagonist on the ovulation triggering day could have a beneficial effect on IVF outcomes, especially oocyte maturity and quality. Pulsatile release of LH by the pituitary was significantly suppressed by the GnRH antagonist for 456 minutes [24]. Stopping of GnRH antagonist administration could immediately reverse the antagonizing effect to GnRH receptors; thus cessation of GnRH antagonist administration on the hCG day appears to partly eliminate the possible detrimental effect of GnRH antagonist on the final oocyte maturation stage.
In contrast, we did not find a significant difference in oocyte or embryo quality in this randomized study. Oocyte quality and the developmental potential of an embryo are strongly associated and it may be assumed that the follicular microenvironment is capable of profoundly influencing the quality of the oocyte obtained at ovulation and the COH outcomes. Therefore, the stimulation protocol used for COH is an important factor in COH. The specific GnRH binding sites have been identified in human granulosa cells [25], therefore GnRH analogues may have a direct effect on the ovary. However, several studies have shown that GnRH analogues have no effect on oocyte or embryo quality. Cota et al. [26] indicated that, in terms of the quality of oocyte morphology, there was no difference between the antagonist multi-dose protocol and the long-term agonist protocol. Another study also showed that there was no difference in the proportion of chromosomally abnormal blastomeres either when using a GnRH agonist or antagonist protocol [27]. Munoz et al. [28] suggested that the type of protocol used for COH influences the kinetics of embryo development, but these variations are not reflected in the embryo quality. Our clinical data also showed that a modified GnRH antagonist protocol did not affect the oocyte or embryo quality. Large scale randomized controlled studies could be needed to identify the effect of GnRH antagonist on oocyte and embryo quality.
Concerns have been raised regarding the possible adverse effects of GnRH antagonist on endometrial receptivity, and these potential effects of GnRH antagonist have been claimed to be causes of a lower pregnancy rate [29,30]. High doses of ganirelix have been connected with low implantation rates [31]. In contrast, when embryos were cryopreserved after an ovulation stimulation cycle in which high dose GnRH antagonists were used and later thawed and transferred, the implantation and pregnancy rates were unaffected by the use of GnRH antagonist during the initial stimulation cycle [30,32]. HOXA10 is a homeobox-containing transcription factor that regulates endometrial development during each menstrual cycle. HOXA 10 expression is necessary for endometrial receptivity. Taylor et al. [33] found that GnRH agonist or GnRH antagonist did not alter endometrial HOXA10 mRNA expression either in vivo or in vitro during COH. Sirayapiwat et al. [34] also reported that GnRH antagonists may have no effect on HOXA10 protein expression in the endometrium obtained during the implantation window of normally menstruating women. On the other hand, GnRH antagonists maybe associated with impaired HOXA10 expression in endometrial stromal cells [35]. Although we did not evaluate HOXA10 expression in endometrial stromal cells, clinical data such as the endometrial thickness, pattern, and implantation rates did not differ between the two groups.
If the ability to prevent a premature LH surge is maintained, adjusting GnRH antagonist administration is a more feasible way than adding other types of gonadotropic drug to prevent drawbacks of the GnRH antagonist protocol. Moreover, the cost of drugs could be reduced by omitting one dose of GnRH antagonist rather than adding exogenous LH. In conclusion, cessation of GnRH antagonist on the hCG trigger day may show comparable COH outcomes when compared to the conventional protocol. In addition, this protocol may reduce the total dose of GnRH antagonist needed for COH and thus reduce the cost of treatment. Our results suggest that this new protocol could be an alternative method for a GnRH antagonist flexible multiple-dose protocol.
Notes
This work was supported by Seoul National University Bundang Hospital grant 11-2009-030.
No potential conflict of interest relevant to this article was reported.