 |
 |
- Search
| Clin Exp Reprod Med > Volume 48(4); 2021 > Article |
|
Abstract
Objective
We investigated the impact of vitamin D3 (VD3) supplementation during mouse preantral follicle culture in vitro and the mRNA expression of 25-hydroxylase (CYP2R1), 1-alpha-hydroxylase (CYP27B1), and vitamin D receptor (VDR) in mouse ovarian follicles at different stages.
Methods
Preantral follicles were retrieved from 39 BDF1 mice (7ŌĆō8 weeks old) and then cultured in vitro for 12 days under VD3 supplementation (0, 25, and 50 pg/mL). Follicular development and the final oocyte acquisition were assessed. Preantral follicles were retrieved from 15 other BDF1 mice (7ŌĆō8 weeks old) and cultured without VD3 supplementation. Three stages of mouse ovarian follicles were obtained (preantral, antral, and ruptured follicles). Total RNA was extracted from the pooled cells (from 20 follicles at each stage), and then reverse transcriptase-polymerase chain reaction was performed to identify mRNA for CYP2R1, CYP27B1, and VDR.
Results
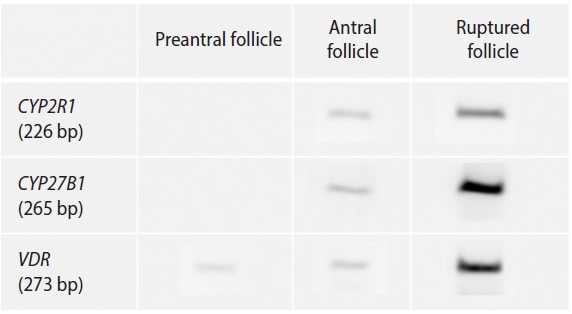
The survival of preantral follicles, rates of antrum formation and ruptured follicles (per initiated follicle) and the number of total or mature oocytes were all comparable among the three groups. Both CYP2R1 and CYP27B1 were expressed in antral and ruptured follicles, but not in preantral follicles. VDR was expressed in all three follicular stages.
Vitamin D (VD) is a steroid hormone involved in calcium and phosphorus homeostasis and bone mineralization [1,2]. VD can be synthesized in the skin through sun exposure or obtained via food intake [1]. The serum level of VD can be affected by age, race, season, and body mass index [2]. Two main enzymes are involved in the synthesis of VD [3]. Hepatic 25-hydroxylase converts VD to 25-hydroxyvitamin D (25OHD, 25-hydroxycholecalciferol; the major circulating form of VD), and renal 1-alpha-hydroxylase converts 25OHD to the active form 1,25-dihyroxyvitamin D3 (1,25(OH)2D3, 1,25-hydroxycholecalciferol, calcitriol, VD3) [4]. CYP2R1 is the most important 25-hydroxylase, and CYP27B1 is the key 1-alpha-hydroxylase. The active form VD3 is the ligand for the vitamin D receptor (VDR), which is present in many female reproductive organs, such as the ovary, endometrium, fallopian tube, and placenta [3]. VDR is present particularly in granulosa cells of the ovarian follicles; therefore, it is believed that VD plays an essential role in ovarian follicular development [3,5,6].
In a human granulosa cell culture model, VD3 supplementation in vitro enhanced 3-beta-hydroxysteroid dehydrogenase activity and progesterone production, but did not affect follicle-stimulating hormone (FSH)-induced aromatase activity and estradiol production [5]. In women with polycystic ovary syndrome, VD supplementation was beneficial for follicular development, with a higher number of dominant follicles and a more regular menstrual cycle [7].
In human in vitro fertilization (IVF) cycles, several studies have reported an association between higher serum or follicular ’¼éuid (FF) 25OHD concentrations and the clinical pregnancy rate; however, other studies have reported contradictory findings [3,6]. Furthermore, it was reported that FF 25OHD levels were inversely related to embryo quality [8,9]. Therefore, there are insufficient data to reach a definitive conclusion regarding the effect of VD supplementation for VD-depleted women undergoing IVF [6]. A few experiments have reported that VD3 supplementation had a direct effect on ovarian follicular development. In goats, VD3 supplementation in vitro during granulosa cell culture decreased the level of reactive oxygen species [10]. In rhesus macaques, VD3 supplementation in vitro increased preantral follicle survival up to the antral follicle stage [11,12].
However, no animal model studies have yet investigated the impact of VD3 supplementation in vitro on the entire folliculogenesis process, up to oocyte acquisition. We investigated whether VD3 supplementation in vitro affected follicular development and final oocyte acquisition in a mouse model of preantral follicle culture. We also investigated whether 25-hydroxylase, 1-alpha-hydroxylase, and VDR were expressed in the three stages of mouse ovarian follicles. If there VD3 supplementation is beneficial for follicular development, a rationale will be provided to recommend sufficient VD intake to improve IVF results.
Female 7- to 8-week old BDF1 mice (Orient Co., Seoul, Korea) were nurtured under 12-hour day and a 12-hour night conditions at 23Ōäā and fed ad libitum. The Institutional Animal Care and Use Committee (IACUC) of Seoul National University Bundang Hospital approved the experiment (No. BA1903-267/014-01). After 1 week of adaptation, 39 mice were killed by cervical dislocation and bilateral ovaries were obtained. They were collected in 1 mL of L-15 medium (WelGENE, Daegu, Korea) supplemented with 0.4% bovine serum albumin (Sigma-Aldrich, St. Louis, MO, USA). From the ovaries, intact preantral follicles were isolated via mechanical tearing by a 1-mL tuberculin syringe [13] and randomly divided into three groups.
Preantral follicles were cultured in a growth medium containing alpha-minimum essential medium (WelGENE), 5% fetal bovine serum (Gibco, Paisley, UK), 10 mIU/mL recombinant FSH (Merck-Serono, Geneva, Switzerland), 1% insulin-transferrin-selenium mixture (Sigma-Aldrich), and 1% penicillin-streptomycin mixture (Sigma-Aldrich). All follicles were cultured in 96-well plates (BD BioCoat; BD Falcon, Franklin Lakes, NJ, USA) at 37Ōäā in 5% CO2 for 10 days. Medium changes were conducted every other day, and follicle survival and antrum formation were assessed. If granulosa cells appeared to be dark and fragmented, they were considered to be dead.
After 10 days of culture in the growth medium, the follicles were transferred to maturation medium and cultured for another 16 hours at 37Ōäā in 5% CO2 to obtain ruptured follicles. The maturation medium was composed of 1.5 IU/mL human chorionic gonadotropin (Merck-Serono), and 5 ng/mL recombinant mouse epidermal growth factor (Sigma-Aldrich) was added to the growth medium. During the 12 days of culture, supplementation with 0, 25, and 50 pg/mL of active-form VD3 (1,25(OH)2D3, Sigma-Aldrich) was performed.
Oocytes were harvested from the finally ruptured (ovulated) follicles. Surrounding cumulus cells were removed by treatment with 0.3% hyaluronidase (Sigma-Aldrich) and gentle pipetting. Oocytes were classified into five categories; germinal vesicle (GV), GV breakdown, metaphase II (MII), degenerated, or dead. If a polar body was present in the perivitelline space, the oocytes were considered to be MII oocytes. Fragmented or dark oocytes were classified as degenerated. If the oocytes were not enclosed by granulosa cells, they were classified as dead. The analysis was performed by one skilled embryologist to minimize inter-observer variability.
From 15 mice, preantral follicles were isolated and cultured in growth medium for 10 days and then transferred to maturation medium for 16 hours, as described above (without VD3 supplementation). Twenty follicles in each stage of folliculogenesis (preantral, antral, and ruptured follicles) were pooled, and the total RNA was extracted using the Dynabeads protocol (Dynabeads mRNA DIRECT Kit; Ambion, Oslo, Norway). The mRNA of CYP2R1 (encoding 25-hydroxylase), CYP27B1 (encoding 1-alpha-hydroxylase), and VDR was transcribed into cDNA using a PrimeScript first-strand cDNA Synthesis Kit (Takara, Kusatsu, Japan) according to the manufacturerŌĆÖs instructions. Each gene was amplified from cDNA using the reverse transcription system: 95Ōäā for 1 minute followed by 35 cycles of 95Ōäā for 15 seconds, 62Ōäā for 15 seconds, and 72Ōäā for 15 seconds. The final extension was at 72Ōäā for 3 minutes. Reverse transcriptase-polymerase chain reaction (RT-PCR) was carried out with a StepOne-Plus real-time PCR system with TaqMan probes (Applied Biosystems, Foster City, CA, USA) in a 20-╬╝L reaction volume containing 10 ╬╝L of Applied Biosystems TaqMan Universal PCR Master Mix I (Cat. no. 4427788), 2 ╬╝L of cDNA, and 6 ╬╝L of RNase-free water. The specific primers are listed in Table 1, and all were purchased from Integrated DNA Technology (Coralville, IA, USA). The amplified RT-PCR products were purified and separated using 2% agarose gel electrophoresis. The assays were repeated three times per sample.
Statistical analyses were performed using IBM SPSS ver. 25.0 (IBM Corp., Armonk, NY, USA). The Fisher exact test was used to compare the rates among the groups. Numerical data were compared using the Kruskal-Wallis test. If the value was significant, the Mann-Whitney U-test with the Bonferroni correction was used for further analysis. A p-value less than 0.05 was considered to indicate statistical significance.
The overall outcomes of in vitro growth of preantral follicles and the percentage of MII oocytes are presented in Table 2. Among the three VD3-supplemented groups (0, 25, and 50 pg/mL), the survival of preantral follicles and the rates of antrum formation or ruptured follicles (per initiated follicle) were all comparable. The number of total oocytes per initiated follicle (80.2%, 81.0%, and 76.2%) and mature oocytes per initiated follicle (24.6%, 27.8%, and 23.0%) were also similar among the three VD3-supplemented groups. Both CYP2R1 and CYP27B1 were expressed in antral and ruptured follicles, but not in preantral follicles (Figure 1). VDR was expressed in all three follicular stages.
In the present work, the expression of mRNA for 25-hydroxylase (CYP2R1) and 1-alpha-hydroxylase (CYP27B1) was observed both in antral follicles and ruptured follicles, but not in preantral follicles. However, mRNA for VDR was expressed in preantral follicles as well as antral and ruptured follicles. It is known that VDR is expressed in mouse ovarian follicles [14]. In that study, however, the follicular stage-specific expression of VDR was not assessed. We obtained preantral follicles as well as antral and ruptured follicles separately, through mouse preantral follicle culture, and confirmed that VDR was expressed in all three stages. Our research is significant because we observed the follicular stage-specific expression of VDR.
Bieche et al. [15] observed that most of the CYP2 family, including CYP2R1 (except CYP2F1), were expressed at variable levels in the human ovary. In that study, however, follicular stage-specific expression of the CYP2 family was not assessed. In the macaque, VDR, CYP2R1, and CYP27B1 were expressed in preantral and small antral follicles [12]. In contrast, we did not observe the expression of CYP2R1 and CYP27B1 mRNA in mouse preantral follicles. The reason for this difference is largely unknown. However, the absence of the two enzymes in mouse preantral follicles does not mean that VD3 has no effect on the growth of mouse preantral follicles. Based on our observations, since VDR is expressed in mouse preantral follicles, we can assume that VD3 can act on preantral follicles in mice. Overall, VDR is expressed in preantral follicles, and CYP2R1 and CYP27B1 are expressed in antral follicles in mice; therefore, the intrinsic VD regulating system is present in the antral follicles.
Nonetheless, VD3 supplementation in vitro (25 or 50 pg/mL) did not enhance the full development of mouse preantral follicles (just up to the ruptured follicle stage) or oocyte acquisition in our study. In a macaque experiment, Xu et al. [11] observed that 25 pg/mL VD3 supplementation in vitro improved preantral follicle survival at week 2, but 100 pg/mL supplementation in vitro did not. Instead, 100 pg/mL VD3 supplementation in vitro led to a larger diameter of antral follicles at week 5. Thus, they argued that VD locally acts on primate follicular development, in a dose- and stage-dependent manner.
The optimal supplemental concentration of VD3 for preantral follicle culture in animals is unknown. Usually, in humans, a serum level of 25OHD (major circulating VD) higher than 30 ng/mL is regarded as replete, and serum level of 25OHD less than 20 ng/mL is regarded as deficient [16]. In the study by Potashnik et al. [17], the average level of 25OHD was 9.1┬▒1.8 ng/mL in human FF and 16.9┬▒1.9 ng/mL in human serum; in contrast, the average level of active VD3 was 22.3┬▒3.4 pg/mL in human FF and 48.5┬▒8.7 pg/mL in human serum at the time of oocyte pickup. Although there have been no reports regarding the appropriate level of active VD3 in mouse FF, we assumed that the 2 VD3 concentrations in our experiment (25 or 50 pg/mL) were sufficient.
In humans, three meta-analyses have reported that women undergoing IVF who have deficient or insufficient 25OHD levels have a lower live birth rate than women who have sufficient 25OHD levels [18-20]. However, the results from individual studies are inconsistent. For example, in a review by Pacis et al. [21], one study demonstrated a negative relationship between VD status and assisted reproductive technology (ART) outcomes, while two studies showed no association; the remaining five studies concluded that ART outcomes improved after VD supplementation. Furthermore, no association of serum 25OHD levels with IVF outcomes, in terms of the clinical pregnancy rate and live birth rate, has yet been reported [22,23]. Moreover, intake of calcitriol (an active form of VD) did not enhance the clinical pregnancy rate of IVF in women with 25OHD deficiency [24]. Therefore, there is no consensus on whether serum 25OHD levels are related to the IVF pregnancy rate, or whether VD supplementation could improve the IVF pregnancy rate in women with 25OHD deficiency.
Measurement of various steroid hormone levels or genes related to the VD3 metabolic pathway after VD3 supplementation would help to elucidate the role of VD3 in folliculogenesis. In a recent study, Makieva et al. [25] found that oral VD supplementation altered the hormonal milieu of FF and the transcriptomic profile of luteinized granulosa cells in women with 25OHD deficiency. They found upregulation of VDR, glutathione-S-transferase A3 (GSTA3), and the interleukin-21 receptor, and downregulation of prostaglandin-endoperoxide synthase 2 (PTGS2), Kruppel-like factor 4 (KLF4), transient receptor potential cation channel subfamily C member (TRPC4), vascular endothelial growth factor, retinoid X receptor beta, and advanced glycation end-product specific receptor by oral VD supplementation. The mechanistic pathway of folliculogenesis after VD3 supplementation in vitro should be further investigated in animal models.
In conclusion, in the current study, we found follicular stage-specific expression of VDR, CYP2R1, and CYP27B1 in mice. Unfortunately, VD3 supplementation in vitro (25 or 50 pg/mL) was not helpful for the full development of preantral follicles and the resultant oocyte acquisition. Further studies are needed to verify whether VD3 supplementation in vitro is effective for the stage-specific development of mouse preantral follicles.
Notes
Figure┬Ā1.
Expression of mRNA for 25-hydroxylase (CYP2R1), 1-alpha-hydroxylase (CYP27B1), and vitamin D receptor (VDR) in the three stages of mouse ovarian follicles.
Table┬Ā1.
Gene primer sequences and their conditions for RT-PCR
Table┬Ā2.
Outcomes of in vitro growth of mice preantral follicle under vitamin D supplementation
References
1. Bouillon R, Carmeliet G, Daci E, Segaert S, Verstuyf A. Vitamin D metabolism and action. Osteoporos Int 1998;8 Suppl 2:S13-9.


2. Wagner CL, Taylor SN, Dawodu A, Johnson DD, Hollis BW. Vitamin D and its role during pregnancy in attaining optimal health of mother and fetus. Nutrients 2012;4:208-30.



3. Anagnostis P, Karras S, Goulis DG. Vitamin D in human reproduction: a narrative review. Int J Clin Pract 2013;67:225-35.


4. Bikle DD. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol 2014;21:319-29.



5. Merhi Z, Doswell A, Krebs K, Cipolla M. Vitamin D alters genes involved in follicular development and steroidogenesis in human cumulus granulosa cells. J Clin Endocrinol Metab 2014;99:E1137-45.



6. Irani M, Merhi Z. Role of vitamin D in ovarian physiology and its implication in reproduction: a systematic review. Fertil Steril 2014;102:460-8.


7. Fang F, Ni K, Cai Y, Shang J, Zhang X, Xiong C. Effect of vitamin D supplementation on polycystic ovary syndrome: a systematic review and meta-analysis of randomized controlled trials. Complement Ther Clin Pract 2017;26:53-60.


8. Anifandis GM, Dafopoulos K, Messini CI, Chalvatzas N, Liakos N, Pournaras S, et al. Prognostic value of follicular fluid 25-OH vitamin D and glucose levels in the IVF outcome. Reprod Biol Endocrinol 2010;8:91.



9. Ciepiela P, Duleba AJ, Kowaleczko E, Chelstowski K, Kurzawa R. Vitamin D as a follicular marker of human oocyte quality and a serum marker of in vitro fertilization outcome. J Assist Reprod Genet 2018;35:1265-76.



10. Yao X, Zhang G, Guo Y, Ei-Samahy M, Wang S, Wan Y, et al. Vitamin D receptor expression and potential role of vitamin D on cell proliferation and steroidogenesis in goat ovarian granulosa cells. Theriogenology 2017;102:162-73.

11. Xu J, Hennebold JD, Seifer DB. Direct vitamin D3 actions on rhesus macaque follicles in three-dimensional culture: assessment of follicle survival, growth, steroid, and antiMullerian hormone production. Fertil Steril 2016;106:1815-20.


12. Xu J, Lawson MS, Xu F, Du Y, Tkachenko OY, Bishop CV, et al. Vitamin D3 regulates follicular development and intrafollicular vitamin D biosynthesis and signaling in the primate ovary. Front Physiol 2018;9:1600.



13. Hong YH, Kim SJ, Kim SK, Lee SC, Jun JH, Jee BC, et al. Impact of imatinib or dasatinib coadministration on in vitro preantral follicle development and oocyte acquisition in cyclophosphamide-treated mice. Clin Exp Reprod Med 2020;47:269-76.



14. Shahbazi M, Jeddi-Tehrani M, Zareie M, Salek-Moghaddam A, Akhondi MM, Bahmanpoor M, et al. Expression profiling of vitamin D receptor in placenta, decidua and ovary of pregnant mice. Placenta 2011;32:657-64.


15. Bieche I, Narjoz C, Asselah T, Vacher S, Marcellin P, Lidereau R, et al. Reverse transcriptase-PCR quantification of mRNA levels from cytochrome (CYP)1, CYP2 and CYP3 families in 22 different human tissues. Pharmacogenet Genomics 2007;17:731-42.


16. Firouzabadi RD, Rahmani E, Rahsepar M, Firouzabadi MM. Value of follicular fluid vitamin D in predicting the pregnancy rate in an IVF program. Arch Gynecol Obstet 2014;289:201-6.


17. Potashnik G, Lunenfeld E, Levitas E, Itskovitz J, Albutiano S, Yankowitz N, et al. The relationship between endogenous oestradiol and vitamin D3 metabolites in serum and follicular fluid during ovarian stimulation for in-vitro fertilization and embryo transfer. Hum Reprod 1992;7:1357-60.


18. Lv SS, Wang JY, Wang XQ, Wang Y, Xu Y. Serum vitamin D status and in vitro fertilization outcomes: a systematic review and meta-analysis. Arch Gynecol Obstet 2016;293:1339-45.


19. Chu J, Gallos I, Tobias A, Tan B, Eapen A, Coomarasamy A. Vitamin D and assisted reproductive treatment outcome: a systematic review and meta-analysis. Hum Reprod 2018;33:65-80.


20. Zhao J, Huang X, Xu B, Yan Y, Zhang Q, Li Y. Whether vitamin D was associated with clinical outcome after IVF/ICSI: a systematic review and meta-analysis. Reprod Biol Endocrinol 2018;16:13.



21. Pacis MM, Fortin CN, Zarek SM, Mumford SL, Segars JH. Vitamin D and assisted reproduction: should vitamin D be routinely screened and repleted prior to ART? A systematic review. J Assist Reprod Genet 2015;32:323-35.


22. Franasiak JM, Molinaro TA, Dubell EK, Scott KL, Ruiz AR, Forman EJ, et al. Vitamin D levels do not affect IVF outcomes following the transfer of euploid blastocysts. Am J Obstet Gynecol 2015;212:315.

23. Cozzolino M, Busnelli A, Pellegrini L, Riviello E, Vitagliano A. How vitamin D level influences in vitro fertilization outcomes: results of a systematic review and meta-analysis. Fertil Steril 2020;114:1014-25.


- TOOLS
-
METRICS

-
- 0 Crossref
- Scopus
- 2,967 View
- 110 Download
- Related articles in Clin Exp Reprod Med
-
Optimization of In Vitro Culture System of Mouse Preantral Follicles.2001 June;28(2)






