 |
 |
- Search
| Clin Exp Reprod Med > Volume 44(1); 2017 > Article |
Abstract
The task force of the Korean Society for Reproductive Immunology recommends intravenous immunoglobulin G treatment in women with reproductive failure, including recurrent pregnancy loss and/or repeated implantation failure, who show cellular immune factors such as abnormal natural killer cell levels, natural killer cell cytotoxicity, and/or type 1 T helper immunity.
The features of the reproductive population in South Korea have been changing: the mean age at first marriage has reached the 30s; the mean age at first pregnancy was 32.2 years in 2015; and those ages have increased continuously (Korean Statistical Information Service, Statistics Korea, 2015). In 2014, the Ministry of Health and Welfare of the Republic of Korea reported that the total number of women with subfertility or infertility had reached 210,000 up from 170,000 in 2007. With the increase in maternal age and the number of infertile women undergoing assisted reproductive interventions, the incidence of reproductive failure, including recurrent pregnancy loss (RPL) and repeated implantation failure (RIF), is increasing.
For a successful pregnancy, the maternal immune system must maintain a balance between defense against microorganisms and accommodation toward a fetus [1]. Immune abnormalities have been reported as an etiology of RPL, RIF, and other pregnancy complications [2,3,4]. Cellular immune abnormalities have been reported in women experiencing reproductive failure, including increased natural killer (NK) cell levels, altered T helper (Th)1/Th2 cell ratios, altered Th17/regulatory T cell ratios, or NK cytotoxicity [1,2,3,4,5,6,7,8]. In addition, the prevalence of antiphospholipid antibodies (APAs) was as high as 20% in women with RPL and 30% in women with RIF [9,10,11,12].
Intravenous immunoglobulin G (IVIG) is derived and purified from the plasma of 3,000 or more donors and contains more than 95% unmodified immunoglobulin G. Since the first demonstration of the effectiveness of IVIG in the treatment of immune thrombocytopenia purpura in 1981 [13], it has been widely used to treat autoimmune and inflammatory diseases, and it was introduced as an intervention for women with RPL in 1991 [14,15,16,17,18]. The immunomodulatory effects of IVIG are mediated through two different portions of the immunoglobulin. The F(ab┬┤)2-dependent mechanisms include the killing of target cells by antibody-dependent cytotoxicity, the blockade of cell-cell interactions mediated by cell-surface receptors such as Fas and Fas ligand (CD95L), the neutralization of cytokines and autoantibodies, and the scavenging of the anaphylatoxins C3a and C5a. The Fc-dependent mechanisms include the saturation of the neonatal Fc receptor, the expansion of regulatory T cell populations, the blockade of immune complex binding to activating Fc╬│ receptors, the modulation of dendritic cell activation via Fc╬│ receptor III, and the modulation of activating and inhibitory Fc╬│ receptor expression on innate
immune effector cells and B cells.
Although the evaluation of cellular immune factors in women with reproductive failure is becoming more popular, appropriate cut-off values for the tests have not yet been established, and remedies for those abnormalities are still controversial. Even though a meta-analysis of IVIG for women with RPL and randomized controlled trials have not demonstrated a beneficial effect [19,20,21], IVIG is commonly prescribed for women with reproductive failure. However, there is no consensus on the indication and treatment protocol for IVIG in those women. Thus the Korean Society for Reproductive Immunology launched the IVIG Task Force to establish IVIG treatment guidelines for women experiencing reproductive failure (Table 1).
We reviewed the relevant scientific papers investigating the clinical efficacy of IVIG for reproductive failure, with a publication date up to September 31, 2016, by searching PubMed, and we determined indications for IVIG treatment for reproductive failure patients. Each indication was classified and assigned a level according to power of the evidence: a level A classification requires at least one randomized control trial as part of a body of literature of overall good quality and consistency that addresses the specific recommendation; level B requires the availability of well-controlled clinical studies, but no randomized control trials on the topic of the recommendation; and level C requires evidence obtained from expert reports of the opinions and/or clinical experiences of respected authorities, indicating an absence of directly applicable clinical studies of good quality [22].
Although RPL has been traditionally defined as three or more consecutive miscarriages [23], the American Society for Reproductive Medicine Practice Committee Opinion defined RPL as two or more failed pregnancies, based on the risk of recurrence and the prevalence of etiologies [24,25]. Unexplained RPL has been defined as those cases without identifiable genetic, uterine, endocrine, infectious, or autoimmune factors [26]. After systematic evaluation, more than half of cases of RPL were classified as unexplained [27]. Several meta-analyses of randomized clinical trials of IVIG therapy for unexplained RPL failed to prove its effectiveness [20,21,28].
Empirical IVIG treatment in women with unexplained RPL is not recommended (evidence level A).
Kwak et al. [29] showed that the use of IVIG in patients with RPL effectively suppressed elevated peripheral blood NK cells (CD56+, CD56+/16+), and 86.3% of women with RPL who had elevated NK cell counts had a successful pregnancy outcome after IVIG and anti-coagulation treatment. Graphou et al. [30] reported favorable effects of IVIG on Th1/Th2 imbalance in women with RPL. Another study also showed that IVIG modifies the Th1/Th2 balance during early pregnancy (4ŌĆō5 gestational weeks) in women with RPL without other etiologies [31]. IVIG was also effective in reducing the increased peripheral blood NK cell counts and/or NK cell activities in women with unexplained RPL [32,33,34]. Moraru et al. [35] reported that IVIG improved the live-birth rate in women with reproductive failure who had high levels of NK cells. Recently, Lee et al. [36] demonstrated that the live-birth rate was significantly higher in cases of idiopathic RPL in women with cellular immune abnormalities treated with IVIG than in cases of idiopathic RPL in women who were not treated with IVIG, as reported in other studies (82% vs. 42%). The possible mechanisms of action of IVIG in RPL are (1) the neutralization of cytotoxic antibodies, (2) the downregulation of NK cell numbers and activity, (3) the regulation of Th1/Th2 balance, and (4) the expansion of regulatory T cell populations [33,37,38].
IVIG treatment is recommended for women who have RPL with cellular immune abnormalities (evidence level A).
A prospective randomized trial reported that low-molecular-weight heparin (LMWH) with low-dose aspirin (LDA) therapy resulted in a higher live-birth rate than IVIG therapy in women with RPL and APAs (84% vs. 57%) [39]. Dendrinos et al. [40] showed consistent results (LMWH with LDA, 72.5% vs. IVIG, 39.5%; p=0.003). Although IVIG has a neutralizing effect for cytotoxic antibodies, the efficacy of IVIG in women with RPL and APAs is not clear based on the published clinical data. In addition, a meta-analysis showed that IVIG treatment had no significant effect on the live-birth rate in women with RPL and APAs [41].
LMWH with LDA should be considered as the standard therapy in cases of RPL with APAs or classic antiphospholipid syndrome (APS), and IVIG is not recommended (evidence level A). However, IVIG could be considered as an alternative treatment in women who suffer side effects from heparin and/or aspirin, or in cases where those medications are contraindicated (evidence level C) [42,43].
RIF is on the rise in the infertile population undergoing in vitro fertilization cycles. Currently, RIF is defined as pregnancy failure even after at least three cycles of embryo transfers of good-quality embryos [44]. Proposed mechanisms for RIF include embryo or endometrial factors, as well as decreased endometrial receptivity, which could be related to uterine anomalies, endometriosis, hydrosalpinx, and thrombophilia [45,46]. Empirical therapy strategies with aspirin and heparin showed no benefit for cases of unexplained RIF [47,48,49]. A randomized clinical trial showed that immunological treatment with IVIG did not improve the live-birth rate in cases of unexplained RIF [50]. Subsequently, a meta-analysis reviewing 10 studies reported that IVIG improved pregnancy outcomes for repeated in vitro fertilization or intracytoplasmic sperm injection failure and unexplained infertility [51]; however, this study included RIF groups both with and without immune abnormalities [35,50,52,53,54,55].
IVIG treatment in cases of unexplained RIF without immune evaluation is not recommended (evidence level A).
The etiologies of RIF overlap with those of RPL, and women who have RIF may experience RPL as well. Increased peripheral blood NK cell counts and NK cell activity were reported in these cases of reproductive failure [56,57,58], as well as unfavorable Th1-oriented changes to NK and NK T-like cells [59]. Thum et al. [56] showed that IVIG suppressed the increased NK cell cytotoxicity in women with a history of RPL or RIF and Heilmann et al. [53] reported that IVIG was beneficial in cases of RIF with high NK cell levels. Moraru et al. [35] also supported the efficacy of IVIG in women with RIF and high levels of NK or NK T-like cells. Subsequently, Winger et al. [60] reported that IVIG significantly improved IVF success rates in subfertile women with increased Th1/Th2 ratios and/or NK cell levels as compared to those who did not undergo the treatment. Another observational study reported that IVIG treatment improved clinical pregnancy and live-birth rates in selected RPL and RIF patients with immunologic alterations [61].
In cases of RIF with cellular immune abnormality, IVIG treatment can be considered (evidence level B).
Approximately 20% of women with RPL have autoimmune issues, including APAs and other autoimmune abnormalities such as anti-nuclear antibodies or thyroid autoantibodies [9,62,63]. As contributing factors for infertility and poor pregnancy outcomes, type 1 diabetes mellitus, systemic lupus erythematosus, rheumatoid arthritis, and other autoimmune disease were reviewed by Carp et al. [64]. One study suggested high-dose IVIG as a safe and effective therapy for pregnant women who had systemic lupus erythematosus and RPL with or without APS [65], but other studies showed that IVIG did not provide a significant benefit in women with APS and/or systemic lupus erythematosus [39,66]. IVIG could improve the disease activity of systemic lupus erythematosus, but there is not enough evidence supporting IVIG use for RPL or RIF patients with autoimmune diseases (evidence level C).
The reported cut-off values of peripheral blood NK cell proportions in cases of reproductive failure differed across studies. Several studies regarded NK cell proportion levels over 12% of peripheral blood mononuclear cells as the cut-off for high NK cell levels, which were associated with poor reproductive outcomes [67]. Another study defined the proportion as over 12.5% [68]. A detailed analysis of cut-off values in cellular immune markers for Korean women with RPL defined proportions of NK cells of over 16.1% as abnormal [8]. A study done in Australia considered abnormal NK cell proportions as >18% [68].
NK cell cytotoxicity is due to the capability of NK cells to lyse other tissues and is measured as the percentage of target cells killed after co-culture with NK cells in vitro [69]. Lee et al. [8] determined the cut-off values of NK cell cytotoxicity and the Th1/Th2 cytokine-producing CD4 cell ratio; the optimal threshold levels of NK cell cytotoxicity were 34.3% at an effector-to-target cell (E:T) ratio of 50:1, 23.8% at an E:T ratio of 25:1, and 9.6% at an E:T ratio of 12.5:1; in addition, the cut-off value of tumor necrosis factor-alpha/interleukin 10 (TNF-╬▒/IL-10)-producing Th cell ratio was 36.2, and it was reported that IVIG therapy improved the live-birth rate in women with RPL and cellular immune abnormalities at or above these cut-off values equally well as in women with RPL without immune abnormalities [36]. Winger et al. [60] showed a significantly improved in vitro fertilization success rate with IVIG in subfertile women with increased Th1/Th2 ratios and/or NK cell counts with the following cut-off values; >30.6 for TNF-╬▒/IL-10 ratios, >20.5 for interferon-╬│/IL-10, and >12% for NK cells.
To evaluate the cellular immune abnormalities in women with reproductive failure, tests for (1) peripheral blood NK cell proportion (evidence level B), (2) NK cytotoxicity (evidence level C), and (3) Th1/Th2 cytokine cell ratios (evidence level B) are recommended (Table 1).
As the half-life of IVIG is 18 to 25 days, it is adequate to administer IVIG every 3 to 4 weeks. To minimize the side effects, the dose of IVIG and infusion rate need to be carefully controlled [70]. The recommended protocol for IVIG is as follows: (1) 400 mg/kg per each treatment, (2) every 3 to 4 weeks, and (3) from the early stage of pregnancy in women with RPL or from the beginning of the in vitro fertilization cycle for RIF patients (evidence level C). The end-point of IVIG treatment and the need for further laboratory tests can be determined by the clinician's decision, depending on the patient's state.
Prior to IVIG infusion in all patients, the blood level of immunoglobulin A must be determined and renal function tests are required. Anaphylactic reactions were reported in immunoglobulin A-deficient patients (<7 mg/dL) with IVIG, and renal insufficiency can be caused by a sugar stabilizer of IVIG that has been associated with high-dose IVIG treatment [71,72,73]. Mild side effects such as fever, malaise, myalgia, and headache were reported in 4% of patients, and myocardial infarction, renal failure, alopecia, aseptic meningitis, and renal necrosis were described as possible severe side effects [74,75,76]. However, with the proper regimen of IVIG in well-selected patients, the occurrence of side effects is very rare.
Data on IVIG treatment in neonates are abundant and there is no report of significant side effects [76,77,78]. Antenatal treatment for fetal neonatal alloimmune thrombocytopenia with maternal IVIG was not associated with lymphocyte activation or premature maturation of the neonatal immune system [79]. In addition, during the past 20 years there have been no reports of significant side effects in mothers or their babies for IVIG use prior to conception and during pregnancy. However, published studies regarding the safety of the fetus with maternal IVIG therapy during pregnancy are lacking, and longitudinal observational studies are needed.
Notes
References
1. Saito S, Nakashima A, Shima T, Ito M. Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. Am J Reprod Immunol 2010;63:601-610.PMID: 20455873.


2. Kwak-Kim JY, Chung-Bang HS, Ng SC, Ntrivalas EI, Mangubat CP, Beaman KD, et al. Increased T helper 1 cytokine responses by circulating T cells are present in women with recurrent pregnancy losses and in infertile women with multiple implantation failures after IVF. Hum Reprod 2003;18:767-773.PMID: 12660269.



3. Aoki K, Kajiura S, Matsumoto Y, Ogasawara M, Okada S, Yagami Y, et al. Preconceptional natural-killer-cell activity as a predictor of miscarriage. Lancet 1995;345:1340-1342.PMID: 7752757.


4. Fukui A, Fujii S, Yamaguchi E, Kimura H, Sato S, Saito Y. Natural killer cell subpopulations and cytotoxicity for infertile patients undergoing in vitro fertilization. Am J Reprod Immunol 1999;41:413-422.PMID: 10392230.


5. Lee SK, Kim JY, Hur SE, Kim CJ, Na BJ, Lee M, et al. An imbalance in interleukin-17-producing T and Foxp3+ regulatory T cells in women with idiopathic recurrent pregnancy loss. Hum Reprod 2011;26:2964-2971.PMID: 21926059.



6. Kwak JY, Beaman KD, Gilman-Sachs A, Ruiz JE, Schewitz D, Beer AE. Up-regulated expression of CD56+, CD56+/CD16+, and CD19+ cells in peripheral blood lymphocytes in pregnant women with recurrent pregnancy losses. Am J Reprod Immunol 1995;34:93-99.PMID: 8526995.


7. Emmer PM, Nelen WL, Steegers EA, Hendriks JC, Veerhoek M, Joosten I. Peripheral natural killer cytotoxicity and CD56(pos) CD16(pos) cells increase during early pregnancy in women with a history of recurrent spontaneous abortion. Hum Reprod 2000;15:1163-1169.PMID: 10783371.



8. Lee SK, Na BJ, Kim JY, Hur SE, Lee M, Gilman-Sachs A, et al. Determination of clinical cellular immune markers in women with recurrent pregnancy loss. Am J Reprod Immunol 2013;70:398-411.PMID: 23656517.


9. Ford HB, Schust DJ. Recurrent pregnancy loss: etiology, diagnosis, and therapy. Rev Obstet Gynecol 2009;2:76-83.PMID: 19609401.


10. Perricone C, De Carolis C, Giacomelli R, Zaccari G, Cipriani P, Bizzi E, et al. High levels of NK cells in the peripheral blood of patients affected with anti-phospholipid syndrome and recurrent spontaneous abortion: a potential new hypothesis. Rheumatology (Oxford) 2007;46:1574-1578.PMID: 17704519.



11. Jaslow CR, Carney JL, Kutteh WH. Diagnostic factors identified in 1020 women with two versus three or more recurrent pregnancy losses. Fertil Steril 2010;93:1234-1243.PMID: 19338986.


12. Buckingham KL, Chamley LW. A critical assessment of the role of antiphospholipid antibodies in infertility. J Reprod Immunol 2009;80:132-145.PMID: 19243840.


13. Imbach P, Barandun S, d'Apuzzo V, Baumgartner C, Hirt A, Morell A, et al. High-dose intravenous gammaglobulin for idiopathic thrombocytopenic purpura in childhood. Lancet 1981;1:1228-1231.PMID: 6112565.


14. Ruiz JE, Cubillos J, Mendoza JC, Espinel FJ, Kwak JY, Beer AE. Autoantibodies to phospholipids and nuclear antigens in non-pregnant and pregnant Colombian women with recurrent spontaneous abortions. J Reprod Immunol 1995;28:41-51.PMID: 7738915.


15. Coulam CB, Krysa L, Stern JJ, Bustillo M. Intravenous immunoglobulin for treatment of recurrent pregnancy loss. Am J Reprod Immunol 1995;34:333-337.PMID: 8607936.


16. Ruiz JE, Kwak JY, Baum L, Gilman-Sachs A, Beaman KD, Kim YB, et al. Intravenous immunoglobulin inhibits natural killer cell activity in vivo in women with recurrent spontaneous abortion. Am J Reprod Immunol 1996;35:370-375.PMID: 8739456.


17. Carp HJ, Ahiron R, Mashiach S, Schonfeld Y, Gazit E, Toder V. Intravenous immunoglobulin in women with five or more abortions. Am J Reprod Immunol 1996;35:360-362.PMID: 8739454.


18. Intravenous immunoglobulin in the prevention of
recurrent miscarriage: the German RSA/IVIG
group. Br J Obstet Gynaecol 1994;101:1072-1077.PMID: 7826961.


19. Stephenson MD, Kutteh WH, Purkiss S, Librach C, Schultz P, Houlihan E, et al. Intravenous immunoglobulin and idiopathic secondary recurrent miscarriage: a multicentered randomized placebo-controlled trial. Hum Reprod 2010;25:2203-2209.PMID: 20634190.



20. Christiansen OB, Larsen EC, Egerup P, Lunoee L, Egestad L, Nielsen HS. Intravenous immunoglobulin treatment for secondary recurrent miscarriage: a randomised, double-blind, placebo-controlled trial. BJOG 2015;122:500-508.PMID: 25412569.


21. Ata B, Tan SL, Shehata F, Holzer H, Buckett W. A systematic review of intravenous immunoglobulin for treatment of unexplained recurrent miscarriage. Fertil Steril 2011;95:1080-1085.e1-2.PMID: 21232738.


22. Fauser BC, Tarlatzis BC, Rebar RW, Legro RS, Balen AH, Lobo R, et al. Consensus on women's health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS consensus workshop group. Fertil Steril 2012;97:28-38.e25.PMID: 22153789.


24. Practice Committee of American Society for Reproductive Medicine. Definitions of infertility and recurrent pregnancy loss: a committee opinion. Fertil Steril 2013;99:63PMID: 23095139.


25. Practice Committee of the American Society for Reproductive Medicine. Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Fertil Steril 2012;98:1103-1111.PMID: 22835448.


26. Stephenson MD. Frequency of factors associated with habitual abortion in 197 couples. Fertil Steril 1996;66:24-29.PMID: 8752606.


27. Tulppala M, Palosuo T, Ramsay T, Miettinen A, Salonen R, Ylikorkala O. A prospective study of 63 couples with a history of recurrent spontaneous abortion: contributing factors and outcome of subsequent pregnancies. Hum Reprod 1993;8:764-770.PMID: 8314975.



28. Hutton B, Sharma R, Fergusson D, Tinmouth A, Hebert P, Jamieson J, et al. Use of intravenous immunoglobulin for treatment of recurrent miscarriage: a systematic review. BJOG 2007;114:134-142.PMID: 17166218.


29. Kwak JY, Kwak FM, Ainbinder SW, Ruiz AM, Beer AE. Elevated peripheral blood natural killer cells are effectively downregulated by immunoglobulin G infusion in women with recurrent spontaneous abortions. Am J Reprod Immunol 1996;35:363-369.PMID: 8739455.


30. Graphou O, Chioti A, Pantazi A, Tsukoura C, Kontopoulou V, Guorgiadou E, et al. Effect of intravenous immunoglobulin treatment on the Th1/Th2 balance in women with recurrent spontaneous abortions. Am J Reprod Immunol 2003;49:21-29.PMID: 12733591.


31. Yamada H, Morikawa M, Furuta I, Kato EH, Shimada S, Iwabuchi K, et al. Intravenous immunoglobulin treatment in women with recurrent abortions: increased cytokine levels and reduced Th1/Th2 lymphocyte ratio in peripheral blood. Am J Reprod Immunol 2003;49:84-89.PMID: 12765346.


32. Perricone R, Di Muzio G, Perricone C, Giacomelli R, De Nardo D, Fontana L, et al. High levels of peripheral blood NK cells in women suffering from recurrent spontaneous abortion are reverted from high-dose intravenous immunoglobulins. Am J Reprod Immunol 2006;55:232-239.PMID: 16451358.


33. Morikawa M, Yamada H, Kato EH, Shimada S, Kishi T, Yamada T, et al. Massive intravenous immunoglobulin treatment in women with four or more recurrent spontaneous abortions of unexplained etiology: down-regulation of NK cell activity and subsets. Am J Reprod Immunol 2001;46:399-404.PMID: 11775009.


34. van den Heuvel MJ, Peralta CG, Hatta K, Han VK, Clark DA. Decline in number of elevated blood CD3(+) CD56(+) NKT cells in response to intravenous immunoglobulin treatment correlates with successful pregnancy. Am J Reprod Immunol 2007;58:447-459.PMID: 17922698.


35. Moraru M, Carbone J, Alecsandru D, Castillo-Rama M, Garcia-Segovia A, Gil J, et al. Intravenous immunoglobulin treatment increased live birth rate in a Spanish cohort of women with recurrent reproductive failure and expanded CD56(+) cells. Am J Reprod Immunol 2012;68:75-84.PMID: 22509929.


36. Lee SK, Kim JY, Han AR, Hur SE, Kim CJ, Kim TH, et al. Intravenous immunoglobulin G improves pregnancy outcome in women with recurrent pregnancy losses with cellular immune abnormalities. Am J Reprod Immunol 2016;75:59-68.PMID: 26510488.


37. Kim DJ, Lee SK, Kim JY, Na BJ, Hur SE, Lee M, et al. Intravenous immunoglobulin G modulates peripheral blood Th17 and Foxp3(+) regulatory T cells in pregnant women with recurrent pregnancy loss. Am J Reprod Immunol 2014;71:441-450.PMID: 24645850.


38. Clark DA, Wong K, Banwatt D, Chen Z, Liu J, Lee L, et al. CD200-dependent and nonCD200-dependent pathways of NK cell suppression by human IVIG. J Assist Reprod Genet 2008;25:67-72.PMID: 18256920.



39. Triolo G, Ferrante A, Ciccia F, Accardo-Palumbo A, Perino A, Castelli A, et al. Randomized study of subcutaneous low molecular weight heparin plus aspirin versus intravenous immunoglobulin in the treatment of recurrent fetal loss associated with antiphospholipid antibodies. Arthritis Rheum 2003;48:728-731.PMID: 12632426.


40. Dendrinos S, Sakkas E, Makrakis E. Low-molecular-weight heparin versus intravenous immunoglobulin for recurrent abortion associated with antiphospholipid antibody syndrome. Int J Gynaecol Obstet 2009;104:223-225.PMID: 19116178.


41. Daya S, Gunby J, Porter F, Scott J, Clark DA. Critical analysis of intravenous immunoglobulin therapy for recurrent miscarriage. Hum Reprod Update 1999;5:475-482.PMID: 10582784.



42. Christiansen OB, Pedersen B, Rosgaard A, Husth M. A randomized, double-blind, placebo-controlled trial of intravenous immunoglobulin in the prevention of recurrent miscarriage: evidence for a therapeutic effect in women with secondary recurrent miscarriage. Hum Reprod 2002;17:809-816.PMID: 11870141.



43. Vaquero E, Lazzarin N, Valensise H, Menghini S, Di Pierro G, Cesa F, et al. Pregnancy outcome in recurrent spontaneous abortion associated with antiphospholipid antibodies: a comparative study of intravenous immunoglobulin versus prednisone plus low-dose aspirin. Am J Reprod Immunol 2001;45:174-179.PMID: 11270643.


44. Margalioth EJ, Ben-Chetrit A, Gal M, Eldar-Geva T. Investigation and treatment of repeated implantation failure following IVF-ET. Hum Reprod 2006;21:3036-3043.PMID: 16905766.



45. Penzias AS. Recurrent IVF failure: other factors. Fertil Steril 2012;97:1033-1038.PMID: 22464759.


46. Coughlan C, Ledger W, Wang Q, Liu F, Demirol A, Gurgan T, et al. Recurrent implantation failure: definition and management. Reprod Biomed Online 2014;28:14-38.PMID: 24269084.


47. Gelbaya TA, Kyrgiou M, Li TC, Stern C, Nardo LG. Low-dose aspirin for in vitro fertilization: a systematic review and meta-analysis. Hum Reprod Update 2007;13:357-364.PMID: 17347160.



48. Dirckx K, Cabri P, Merien A, Galajdova L, Gerris J, Dhont M, et al. Does low-dose aspirin improve pregnancy rate in IVF/ICSI? A randomized double-blind placebo controlled trial. Hum Reprod 2009;24:856-860.PMID: 19131401.



49. Stern C, Chamley L, Norris H, Hale L, Baker HW. A randomized, double-blind, placebo-controlled trial of heparin and aspirin for women with in vitro fertilization implantation failure and antiphospholipid or antinuclear antibodies. Fertil Steril 2003;80:376-383.PMID: 12909502.


50. Stephenson MD, Fluker MR. Treatment of repeated unexplained in vitro fertilization failure with intravenous immunoglobulin: a randomized, placebo-controlled Canadian trial. Fertil Steril 2000;74:1108-1113.PMID: 11119735.


51. Li J, Chen Y, Liu C, Hu Y, Li L. Intravenous immunoglobulin treatment for repeated IVF/ICSI failure and unexplained infertility: a systematic review and a meta-analysis. Am J Reprod Immunol 2013;70:434-447.PMID: 24238107.


52. Virro MR, Winger EE, Reed JL. Intravenous immunoglobulin for repeated IVF failure and unexplained infertility. Am J Reprod Immunol 2012;68:218-225.PMID: 22805355.


53. Heilmann L, Schorsch M, Hahn T. CD3ŌłÆCD56+CD16+ natural killer cells and improvement of pregnancy outcome in IVF/ICSI failure after additional IVIG-treatment. Am J Reprod Immunol 2010;63:263-265.PMID: 20064143.


54. Coulam CB, Goodman C. Increased pregnancy rates after IVF/ET with intravenous immunoglobulin treatment in women with elevated circulating C56+ cells. Early Pregnancy 2000;4:90-98.PMID: 11723539.

55. De Placido G, Zullo F, Mollo A, Cappiello F, Nazzaro A, Colacurci N, et al. Intravenous immunoglobulin (IVIG) in the prevention of implantation failures. Ann N Y Acad Sci 1994;734:232-234.PMID: 7978921.


56. Thum MY, Bhaskaran S, Abdalla HI, Ford B, Sumar N, Bansal A. Prednisolone suppresses NK cell cytotoxicity in vitro in women with a history of infertility and elevated NK cell cytotoxicity. Am J Reprod Immunol 2008;59:259-265.PMID: 18275519.


57. Thum MY, Bhaskaran S, Abdalla HI, Ford B, Sumar N, Shehata H, et al. An increase in the absolute count of CD56dim CD16+ CD69+ NK cells in the peripheral blood is associated with a poorer IVF treatment and pregnancy outcome. Hum Reprod 2004;19:2395-2400.PMID: 15319390.



58. Rai R, Sacks G, Trew G. Natural killer cells and reproductive failure: theory, practice and prejudice. Hum Reprod 2005;20:1123-1126.PMID: 15760961.



59. Miko E, Manfai Z, Meggyes M, Barakonyi A, Wilhelm F, Varnagy A, et al. Possible role of natural killer and natural killer T-like cells in implantation failure after IVF. Reprod Biomed Online 2010;21:750-756.PMID: 21051289.


60. Winger EE, Reed JL, Ashoush S, El-Toukhy T, Ahuja S, Taranissi M. Elevated preconception CD56+ 16+ and/or Th1:Th2 levels predict benefit from IVIG therapy in subfertile women undergoing IVF. Am J Reprod Immunol 2011;66:394-403.PMID: 21623994.


61. Ramos-Medina R, Garcia-Segovia A, Gil J, Carbone J, Aguaron de la Cruz A, Seyfferth A, et al. Experience in IVIg therapy for selected women with recurrent reproductive failure and NK cell expansion. Am J Reprod Immunol 2014;71:458-466.PMID: 24612159.


62. Thangaratinam S, Tan A, Knox E, Kilby MD, Franklyn J, Coomarasamy A. Association between thyroid autoantibodies and miscarriage and preterm birth: meta-analysis of evidence. BMJ 2011;342:d2616PMID: 21558126.



63. Ticconi C, Rotondi F, Veglia M, Pietropolli A, Bernardini S, Ria F, et al. Antinuclear autoantibodies in women with recurrent pregnancy loss. Am J Reprod Immunol 2010;64:384-392.PMID: 20482520.


64. Carp HJ, Selmi C, Shoenfeld Y. The autoimmune bases of infertility and pregnancy loss. J Autoimmun 2012;38:J266-J274.PMID: 22284905.


65. Perricone R, De Carolis C, Kroegler B, Greco E, Giacomelli R, Cipriani P, et al. Intravenous immunoglobulin therapy in pregnant patients affected with systemic lupus erythematosus and recurrent spontaneous abortion. Rheumatology (Oxford) 2008;47:646-651.PMID: 18346976.



66. Spinnato JA, Clark AL, Pierangeli SS, Harris EN. Intravenous immunoglobulin therapy for the antiphospholipid syndrome in pregnancy. Am J Obstet Gynecol 1995;172(2 Pt 1): 690-694.PMID: 7856708.


67. Beer AE, Kwak JY, Ruiz JE. Immunophenotypic profiles of peripheral blood lymphocytes in women with recurrent pregnancy losses and in infertile women with multiple failed in vitro fertilization cycles. Am J Reprod Immunol 1996;35:376-382.PMID: 8739457.


68. King K, Smith S, Chapman M, Sacks G. Detailed analysis of peripheral blood natural killer (NK) cells in women with recurrent miscarriage. Hum Reprod 2010;25:52-58.PMID: 19819893.



69. Kwak JY, Gilman-Sachs A, Moretti M, Beaman KD, Beer AE. Natural killer cell cytotoxicity and paternal lymphocyte immunization in women with recurrent spontaneous abortions. Am J Reprod Immunol 1998;40:352-358.PMID: 9870079.


70. Sherer Y, Levy Y, Shoenfeld Y. IVIG in autoimmunity and cancer: efficacy versus safety. Expert Opin Drug Saf 2002;1:153-158.PMID: 12904149.


71. Burks AW, Sampson HA, Buckley RH. Anaphylactic reactions after gamma globulin administration in patients with hypogammaglobulinemia: detection of IgE antibodies to IgA. N Engl J Med 1986;314:560-564.PMID: 3945295.


72. Rachid R, Bonilla FA. The role of anti-IgA antibodies in causing adverse reactions to gamma globulin infusion in immunodeficient patients: a comprehensive review of the literature. J Allergy Clin Immunol 2012;129:628-634.PMID: 21835445.


73. Zhang R, Szerlip HM. Reemergence of sucrose nephropathy: acute renal failure caused by high-dose intravenous immune globulin therapy. South Med J 2000;93:901-904.PMID: 11005352.

74. Thornton CA, Ballow M. Safety of intravenous immunoglobulin. Arch Neurol 1993;50:135-136.PMID: 8431130.


75. Rauova L, Rovensky J, Shoenfeld Y. High dose intravenous immunoglobulins: a new step in the treatment of systemic lupus erythematosus. Isr Med Assoc J 2000;2:388-392.PMID: 10892397.

76. Dalakas MC. Intravenous immune globulin therapy for neurologic diseases. Ann Intern Med 1997;126:721-730.PMID: 9139559.


77. Kinney J, Mundorf L, Gleason C, Lee C, Townsend T, Thibault R, et al. Efficacy and pharmacokinetics of intravenous immune globulin administration to high-risk neonates. Am J Dis Child 1991;145:1233-1238.PMID: 1951212.


78. Ohlsson A, Lacy J. Intravenous immunoglobulin for suspected or subsequently proven infection in neonates. Cochrane Database Syst Rev 2010;(3): CD001239PMID: 20238315.


79. Radder CM, Roelen DL, van de Meer-Prins EM, Claas FH, Kanhai HH, Brand A. The immunologic profile of infants born after maternal immunoglobulin treatment and intrauterine platelet transfusions for fetal/neonatal alloimmune thrombocytopenia. Am J Obstet Gynecol 2004;191:815-820.PMID: 15467547.


Table┬Ā1
Korean Society for Reproductive Immunology guidelines for IVIG treatment for women experiencing reproductive failure
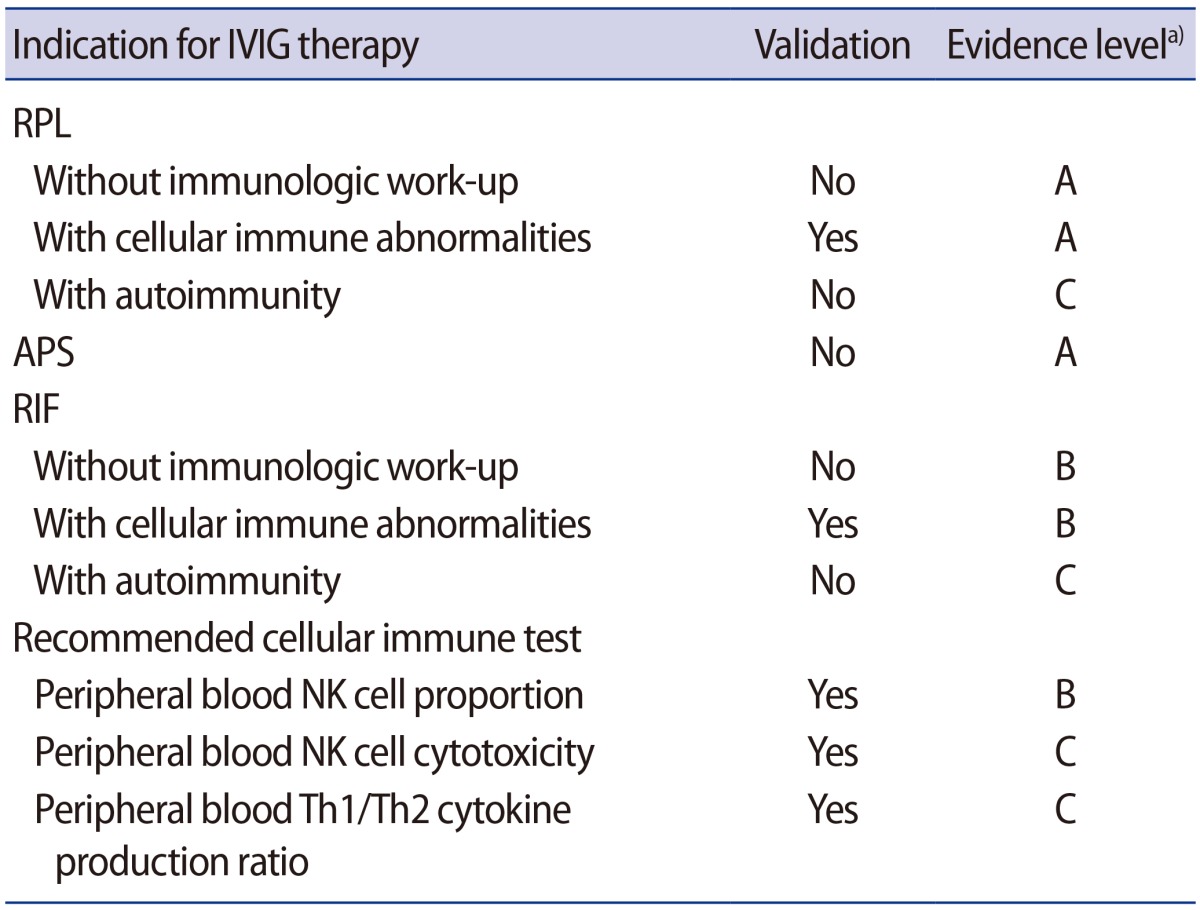
IVIG, intravenous immunoglobulin G; RPL, recurrent pregnancy loss; APS, antiphospholipid syndrome; RIF, repeated implantation failure; NK, natural killer; Th, T helper.
a)Level A, based on meta-analysis and/or randomized controlled trials; level B, no randomized control trials and based on case-control studies; level C, expert opinion and/or clinical experiences of respected authorities.






