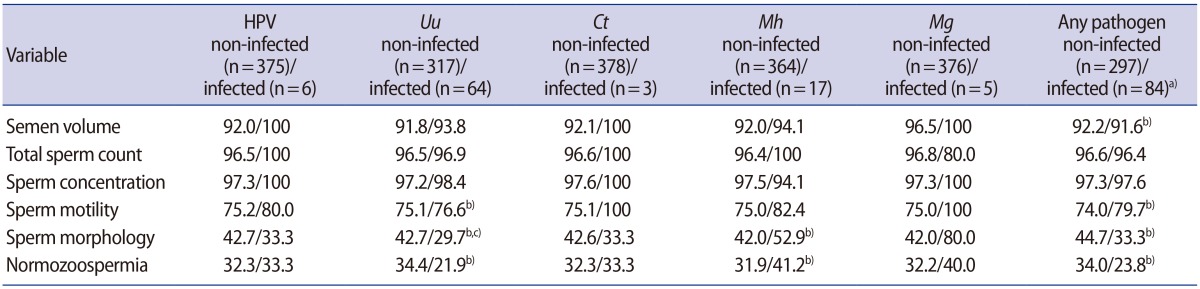
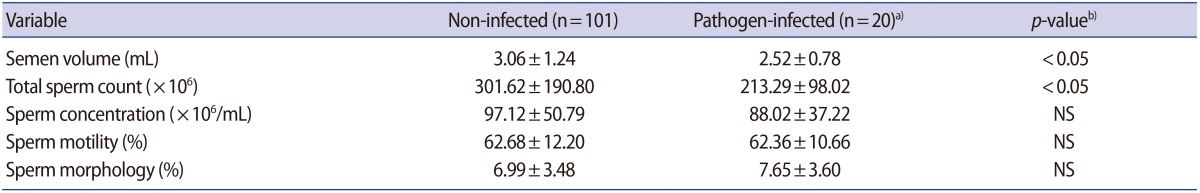
1. Centers for Disease Control and Prevention (CDC). STD-prevention counseling practices and human papillomavirus opinions among clinicians with adolescent patients: United States, 2004. MMWR Morb Mortal Wkly Rep 2006;55:1118-1120.PMID:
17060897.


2. World Health Organization. Sexually transmitted infections (STIs): fact sheet. Geneva: World Health Organization; 2016.
3. Schiffman M, Castle PE. Human papillomavirus: epidemiology and public health. Arch Pathol Lab Med 2003;127:930-934.PMID:
12873163.



4. Gross G, Pfister H. Role of human papillomavirus in penile cancer, penile intraepithelial squamous cell neoplasias and in genital warts. Med Microbiol Immunol 2004;193:35-44.PMID:
12838415.


5. Yang Y, Jia CW, Ma YM, Zhou LY, Wang SY. Correlation between HPV sperm infection and male infertility. Asian J Androl 2013;15:529-532.PMID:
23603919.



6. Foresta C, Noventa M, De Toni L, Gizzo S, Garolla A. HPV-DNA sperm infection and infertility: from a systematic literature review to a possible clinical management proposal. Andrology 2015;3:163-173.PMID:
25270519.


8. Witkin SS, Kligman I, Bongiovanni AM. Relationship between an asymptomatic male genital tract exposure to Chlamydia trachomatis and an autoimmune response to spermatozoa. Hum Reprod 1995;10:2952-2955.PMID:
8747052.



9. Karinen L, Pouta A, Hartikainen AL, Bloigu A, Paldanius M, Leinonen M, et al. Association between Chlamydia trachomatis antibodies and subfertility in the Northern Finland Birth Cohort 1966 (NFBC 1966), at the age of 31 years. Epidemiol Infect 2004;132:977-984.PMID:
15473162.



11. Hosseinzadeh S, Brewis IA, Eley A, Pacey AA. Co-incubation of human spermatozoa with Chlamydia trachomatis serovar E causes premature sperm death. Hum Reprod 2001;16:293-299.PMID:
11157823.



12. Eley A, Hosseinzadeh S, Hakimi H, Geary I, Pacey AA. Apoptosis of ejaculated human sperm is induced by co-incubation with Chlamydia trachomatis lipopolysaccharide. Hum Reprod 2005;20:2601-2607.PMID:
15905291.



13. Xu C, Sun GF, Zhu YF, Wang YF. The correlation of Ureaplasma urealyticum infection with infertility. Andrologia 1997;29:219-226.PMID:
9263572.


14. Lee JS, Kim KT, Lee HS, Yang KM, Seo JT, Choe JH. Concordance of Ureaplasma urealyticum and Mycoplasma hominis in infertile couples: impact on semen parameters. Urology 2013;81:1219-1224.PMID:
23602797.


15. Al-Sweih NA, Al-Fadli AH, Omu AE, Rotimi VO. Prevalence of Chlamydia trachomatis, Mycoplasma hominis, Mycoplasma genitalium, and Ureaplasma urealyticum infections and seminal quality in infertile and fertile men in Kuwait. J Androl 2012;33:1323-1329.PMID:
22052774.


16. Rodriguez R, Hernandez R, Fuster F, Torres A, Prieto P, Alberto J. Genital infection and infertility. Enferm Infecc Microbiol Clin 2001;19:261-266.PMID:
11440663.


17. Dunne EF, Nielson CM, Stone KM, Markowitz LE, Giuliano AR. Prevalence of HPV infection among men: a systematic review of the literature. J Infect Dis 2006;194:1044-1057.PMID:
16991079.



18. Foresta C, Garolla A, Zuccarello D, Pizzol D, Moretti A, Barzon L, et al. Human papillomavirus found in sperm head of young adult males affects the progressive motility. Fertil Steril 2010;93:802-806.PMID:
19100537.


19. Palefsky JM. Human papillomavirus-related disease in men: not just a women's issue. J Adolesc Health 2010;46(4 Suppl): S12-S19.PMID:
20307839.



20. Nielson CM, Flores R, Harris RB, Abrahamsen M, Papenfuss MR, Dunne EF, et al. Human papillomavirus prevalence and type distribution in male anogenital sites and semen. Cancer Epidemiol Biomarkers Prev 2007;16:1107-1114.PMID:
17548671.


21. Gimenes F, Souza RP, Bento JC, Teixeira JJ, Maria-Engler SS, Bonini MG, et al. Male infertility: a public health issue caused by sexually transmitted pathogens. Nat Rev Urol 2014;11:672-687.PMID:
25330794.



22. Brookings C, Goldmeier D, Sadeghi-Nejad H. Sexually transmitted infections and sexual function in relation to male fertility. Korean J Urol 2013;54:149-156.PMID:
23526114.



23. Huang C, Zhu HL, Xu KR, Wang SY, Fan LQ, Zhu WB. Mycoplasma and Ureaplasma infection and male infertility: a systematic review and meta-analysis. Andrology 2015;3:809-816.PMID:
26311339.


24. Liu J, Wang Q, Ji X, Guo S, Dai Y, Zhang Z, et al. Prevalence of Ureaplasma urealyticum, Mycoplasma hominis, Chlamydia trachomatis infections, and semen quality in infertile and fertile men in China. Urology 2014;83:795-799.PMID:
24411218.


25. Seo HH, Kim YJ, Jeong MS, Hong SR, Lee IH, So KA, et al. Combined SYBR green real-time polymerase chain reaction and microarray method for the simultaneous determination of human papillomavirus loads and genotypes. Obstet Gynecol Sci 2016;59:489-497.PMID:
27896251.



26. Park HR, Kim YH, Lee HJ, Oh JS, Kim HJ. Usefulness of the MYCOFAST test (MYCOFAST® Evolution 2) for the diagnosis of non-gonococcal genitourinary infections. Korean J Urol 2006;47:1117-1123.

27. Shin HR, Franceschi S, Vaccarella S, Roh JW, Ju YH, Oh JK, et al. Prevalence and determinants of genital infection with papillomavirus, in female and male university students in Busan, South Korea. J Infect Dis 2004;190:468-476.PMID:
15243918.



28. Castellsague X. Natural history and epidemiology of HPV infection and cervical cancer. Gynecol Oncol 2008;110(3 Suppl 2): S4-S7.PMID:
18760711.


29. Huang C, Long X, Jing S, Fan L, Xu K, Wang S, et al. Ureaplasma urealyticum and Mycoplasma hominis infections and semen quality in 19,098 infertile men in China. World J Urol 2016;34:1039-1044.PMID:
26537883.



30. Brossfield JE, Chan PJ, Patton WC, King A. Tenacity of exogenous human papillomavirus DNA in sperm washing. J Assist Reprod Genet 1999;16:325-328.PMID:
10394529.



31. Connelly DA, Chan PJ, Patton WC, King A. Human sperm deoxyribonucleic acid fragmentation by specific types of papillomavirus. Am J Obstet Gynecol 2001;184:1068-1070.PMID:
11349159.


32. Rintala MA, Grenman SE, Pollanen PP, Suominen JJ, Syrjanen SM. Detection of high-risk HPV DNA in semen and its association with the quality of semen. Int J STD AIDS 2004;15:740-743.PMID:
15537460.


33. Motrich RD, Cuffini C, Oberti JP, Maccioni M, Rivero VE. Chlamydia trachomatis occurrence and its impact on sperm quality in chronic prostatitis patients. J Infect 2006;53:175-183.PMID:
16376990.


34. Puerta Suarez J, Sanchez LR, Salazar FC, Saka HA, Molina R, Tissera A, et al. Chlamydia trachomatis neither exerts deleterious effects on spermatozoa nor impairs male fertility. Sci Rep 2017;7:1126PMID:
28442719.



35. Gerovassili A, Marcandona O, Asimakopoulos B, Karavasilis V, Panopoulou M, Ikonomidis A. Relationship between Chlamydia-Ureaplasma-Mycoplasma genital detection with semen concentration and motility among Greek men. Int J Fertil Steril 2017;11:130-133.PMID:
28670432.


36. Styler M, Shapiro SS. Mollicutes (mycoplasma) in infertility. Fertil Steril 1985;44:1-12.PMID:
3891422.


37. Bliatka D, Lymperi S, Mastorakos G, Goulis DG. Effect of endocrine disruptors on male reproduction in humans: why the evidence is still lacking? Andrology 2017;5:404-407.PMID:
28296338.


38. Comhaire FH, de Kretser D, Farley TM, Rowe PJ. Towards more objectivity in diagnosis and management of male infertility. Int J Androl 1987;7:1-53.
39. World Health Organization. WHO manual for the standardized investigation and diagnosis of the infertile couple. Geneva: World Health Organization; 2000.