 |
 |
- Search
| Clin Exp Reprod Med > Volume 40(2); 2013 > Article |
Abstract
Objective
Impairment of spermatogenesis has been identified as an inevitable side effect of cancer treatment. Although estrogen treatment stimulates spermatogenic recovery from the impaired spermatogenesis by suppressing the intra-testicular testosterone (ITT) level, side effects of estrogen are still major impediments to its clinical application in humans. Soybeans are rich in genistein, which is a phytoestrogen that binds to estrogen receptors and has an estrogenic effect. We investigated the effects of genistein administration on ITT levels, testis weight, and recovery of spermatogenesis in rats treated with a chemotherapeutic agent, busulfan.
Methods
Busulfan was administered intraperitoneally to rats, and then a GnRH agonist was injected subcutaneously into the back, or genistein was administered orally.
Results
The weight of the testes was significantly reduced by the treatment with busulfan. The testis weight was partially restored after busulfan treatment by additional treatment with either the GnRH agonist or genistein. Busulfan also induced atrophy of a high percentage of the seminiferous tubules, but this percentage was decreased by additional treatment with either the GnRH agonist or genistein. Treatment with genistein was effective at suppressing and maintaining ITT levels comparable to that in the GnRH agonist group.
Conclusion
Genistein effectively suppressed ITT levels and stimulated the recovery of spermatogenesis in rats treated with a chemotherapeutic drug. This suggests that genistein may be a substitute for estrogens, for helping humans to recover fertility after cancer therapy without the risk of side effects.
Impairment of spermatogenesis as a result of treatment with chemotherapeutic drugs or radiation has been identified as an inevitable side effect of cancer treatment in human. Previous studies using a rat model have shown that spermatogenesis impaired by radiation or chemotherapy can be restored by treatment with GnRH agonists [1-3] or antagonists [4-6] administered either before [7,8] or after [6,9] the cancer therapy. One of the proposed mechanisms underlying GnRH-analog-induced restoration of spermatogenesis is suppression of testosterone and follicle stimulating hormone [10-13]; another, more recently suggested potential mechanism is suppression of intra-testicular testosterone (ITT) levels [14]. Under normal conditions, high ITT levels are essential for maintaining spermatogenesis, whereas they inhibit spermatogonial differentiation under chemotherapy-induced pathological conditions. A previous study found that exogenous estrogen stimulated spermatogenic recovery of irradiated rats by suppressing ITT [15].
However, the side effects of estrogen treatment, such as gynecomastia and cardiovascular problems [16], represent major impediments to its clinical application. Genistein is a phytoestrogen (an estrogen-like chemical compound present in plants) that binds to estrogen receptors [17-19] and exerts both weak estrogenic and antiestrogenic effects [20]. Exposure to low doses of phytoestrogens in the perinatal period affects Leydig cell function in adult rats, causing a decrease in testicular testosterone secretion [21]. Moreover, unlike estrogen, genistein prevents breast [22,23] and prostate cancer [24,25] and exhibits antitumor activity [26-28].
In the present study, we investigated the effects of genistein administration on ITT levels, testis weight, and recovery of spermatogenesis in rats treated with a chemotherapeutic agent, busulfan, to evaluate the possibility of the clinical application of genistein.
All animal housing and surgical procedures were carried out in accordance with the guidelines of the Institutional Animal Care and Use Committee of MizMedi Hospital, Seoul, Korea (Miz-ani-IRB 20090715).
A total of 150 male Sprague-Dawley rats were used for the present study, and they were maintained on a 12 hours light/dark cycle and were allowed food and water ad libitum. All of the rats were acclimatized for at least 7 days before the initiation of experiments, at which time they were 7 weeks old.
Busulfan (Sigma, St. Louis, MO, USA), an anticancer drug, was first dissolved in a 100% dimethyl sulfoxide solution (8 mg/mL). The same volume of distilled water was added just before use to give a final concentration of busulfan (4 mg/mL). This busulfan solution was administered intraperitoneally to the rats at a dose of 25 mg/1 kg of body weight. When the body weight of the rat was 200 g, 5 mg (1.25 mL) busulfan was administered.
A GnRH agonist (leuprorelin) was obtained from Dong-gook Pharmaceutical (Seoul, Korea). GnRH injections were prepared by suspending a vial of 2.6 mg of GnRH into 1.6 mL of its solvent. 0.65 mg of the GnRH solution (0.4 mL) was injected subcutaneously into the back of the rat.
Genistein was purchased from LC Laboratories (Woburn, MA, USA), and was administered daily to the rats orally at a dose of either 50 or 100 mg/kg with a oral zonde for a period of 4 weeks (from 3 weeks to 7 weeks) (Figure 1).
One hundred fifty rats were divided into 5 groups, with 30 in each group; 1) control, 2) busulfan, 3) busulfan+GnRH, 4) busulfan+genistein (50 mg), and 5) busulfan+genistein (100 mg). The experimental design is shown schematically in Figure 1.
All of the rats (with the exception of the control group) were first weighed before being given a single injection of busulfan at experimental week 0. At week 3 and thereafter, either a single injection of the GnRH agonist was administered, or daily oral administration of genistein was initiated (depending upon the experimental group).
All of the animals were sacrificed by CO2 asphyxiation at experimental week 13. Both testes were freed of the tunica, weighed, and one testis was homogenized in 2 mL of cold water. The testicular supernatant in the homogenate was stored at -80Ōäā for ITT analysis. The level of ITT was assayed in samples from experiments by using T-antiserum-coated tubes (Diagnostic Systems Laboratories, Webster, TX, USA). The ITT level was estimated by chemiluminescence immunoassay and expressed as the amount per gram of testis, to reflect the actual concentration of testosterone to which the testicular cells were exposed [5].
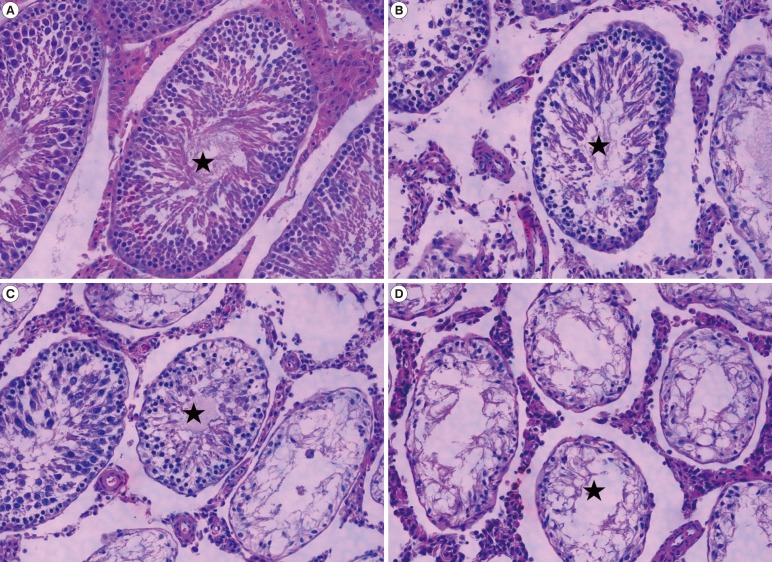
Histological analysis of the testes was performed at experimental week 13. The testes were fixed in Bouin's fixative, dehydrated, and then embedded in paraffin. Thereafter, 5 ┬Ąm-thick serial sections were prepared, and at least five slides from each testis were stained with hematoxylin and eosin for histological assessment. A single slice from each testis was examined under a light microscope. The condition of spermatogenesis in each seminiferous tubule was classified as follows according to the number of germ cell layers present: 'recovery' (at least five layers), 'half recovery' (three or four layers), 'poor recovery' (one or two layers), and 'atrophy' (no germ cells).
The statistically significant differences among the various treatment groups were determined using one-way ANOVA. All statistical analyses were performed using SPSS ver. 16.0 (SPSS Inc., Chicago, IL, USA). For the statistical procedure performed, a p-value<0.05 was considered significant.
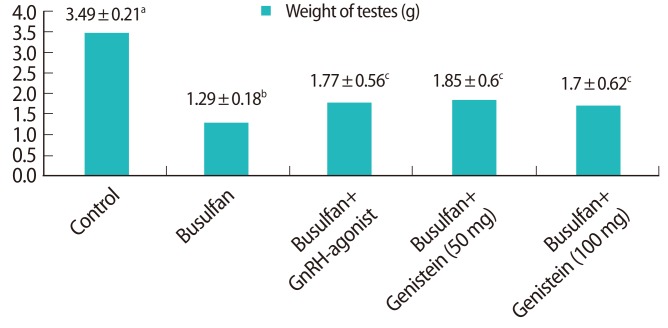
The testes appeared to be significantly smaller in the groups treated with busulfan than in the control group. The testes were weighed bilaterally in all groups (Figure 2). The weights of the testes were significantly decreased in the busulfan (36%, 1.29 g), busulfan+GnRH agonist (50%, 1.77 g), busulfan+genistein (50 mg) (53%, 1.85 g), and busulfan+genistein (100 mg) (48%, 1.70 g) groups compared to the control group (100%, 3.49 g, p<0.05). The testis weight was partially restored after treatment with busulfan by additional treatment with either the GnRH agonist or genistein. The testis weight did not differ between the GnRH agonist and genistein treatment groups.
Seminiferous tubule cross-sections were observed and then classified as follows according to the number of germ cell layers present: 'recovery' (at least five layers), 'half recovery' (three or four layers), 'poor recovery' (one or two layers), and 'atrophy' (no germ cells). For each rat, approximately 300 seminiferous tubules were evaluated in this way (Figure 3).
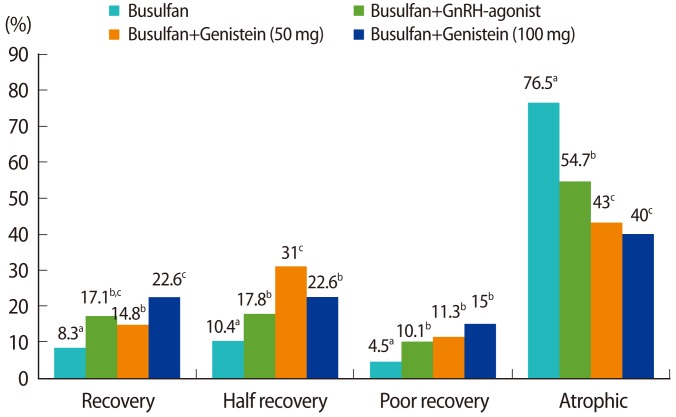
The effect of genistein treatment on the recovery of seminiferous tubules treated with busulfan was investigated (Figure 4). Busulfan-induced cytotoxicity resulted in atrophy of a high percentage of the seminiferous tubules (76.5%, p<0.05), but this percentage was significantly reduced by additional treatment with either the GnRH agonist (54.7%) or genistein (40% to 43%). Moreover, the percentage of 'recovery' tubules was significantly higher in animals treated with the GnRH agonist (17.1%), genistein (50 mg) (14.8%), and genistein (100 mg) (22.6%) than in the busulfan group (8.3%, p<0.05). Histological analysis revealed that many tubules from the busulfan-treated rats contained no detectable spermatozoa and were devoid of germ cells. Although there was no significant difference in the recovery of spermatogenesis between the GnRH agonist and genistein groups, treatment with 100 mg of genistein seemed to be the most effective at restoring the spermatogenesis of seminiferous tubules damaged by busulfan.
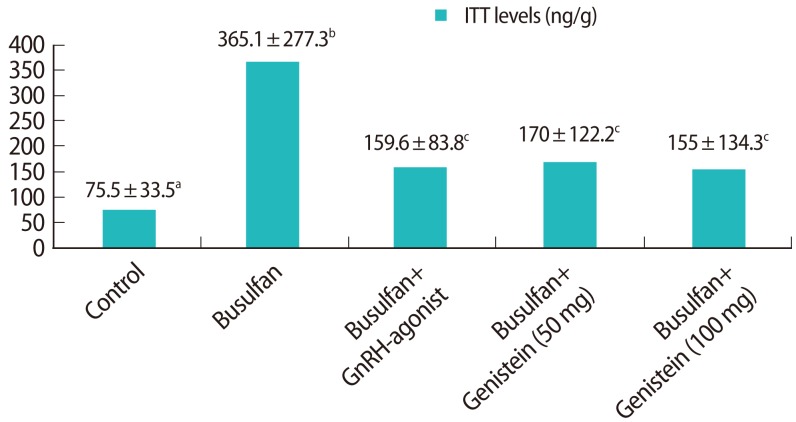
ITT levels per gram of testis were assessed at experimental week 13 (Figure 5). The ITT level was significantly higher in the busulfan group (365.1┬▒277.3 ng/g, p<0.05) than the control group (75.5┬▒33.5 ng/g). The ITT levels of the busulfan+GnRH agonist (159.6┬▒83.8 ng/g) and busulfan+genistein (100 mg) (155.0┬▒134.3 ng/g) groups did not differ from that in the control group, but the level in the busulfan+genistein (50 mg) group (170.0┬▒122.2 ng/g, p<0.05) was significantly higher than in the control group. This indicates that both the 50 mg and 100 mg of genistein treatments were effective at suppressing and maintaining ITT comparable to GnRH agonist treatment.
In the present study, we selected busulfan as a chemotherapy drug, because it is a potent agent that preferentially kills spermatogonial stem cells [29], unlike other chemotherapeutics that destroy differentiated spermatogonia. The testes were significantly smaller in the rats treated with busulfan than in the control group, which is consistent with the findings of a previous study [9]. The diameter of the seminiferous tubules was significantly decreased by busulfan treatment, probably as a result of spermatogenic cell loss [9,30]. The busulfan-induced decreased weight of the testis was partially recovered by additional treatment with either the GnRH agonist or genistein. It has been reported previously that this damage induced by busulfan is dose-dependent and reversible [31-33]; however, this did not seem to be the case in the present study. The weight of the testes was significantly recovered only by additional treatment with either the GnRH agonist or genistein.
Histological analysis of the testes revealed that the testes of rats treated with busulfan exhibited atrophy of the seminiferous tubules, which contained no apparent spermatozoa and were devoid of germ cells. This concurs with the findings of a previous study, although the percentages of 'recovery' and 'atrophy' tubules differ somewhat between the studies. The cytotoxicity of busulfan was significantly reduced by additional treatment with the GnRH agonist, which resulted in a partial recovery of spermatogenesis in the seminiferous tubules. The effect of the GnRH agonist on the recovery of spermatogenesis in testes treated with busulfan has already been reported [9].
While there have been no report regarding the effect of genistein on the cytotoxicity of busulfan, we observed herein that genistein treatment also significantly reduced the cytotoxicity of busulfan and induced the recovery of spermatogenesis. Genistein is one of the phytoestrogens (a group of natural selective estrogen receptor modulators, nonsteroidal, diphenolic structures found in many plants, especially soy products) that binds to estrogen receptors [17-19] and has physiological characteristics similar to those of endogenous estrogens [19,34]. It has been found that estrogen treatment alone after irradiation stimulated spermatogenic recovery [15], and further, that it stimulated the GnRH-antagonist-induced spermatogenic recovery in irradiated rats [35,36]. These reports support and explain our finding that genistein treatment stimulates the recovery of spermatogenesis in rats treated with a chemotherapeutic drug.
It has been noted that ITT levels are elevated after irradiation or chemical treatments, which suggests that an excess of ITT is detrimental to spermatogenesis. ITT levels are suppressed by GnRH analogs that interrupt the pituitary-gonadal axis through flare-up and down-regulation [9]. In the present study, we observed that genistein also effectively suppressed ITT levels. Genistein has similar characteristics to estrogens, acting on Leydig cells to down-regulate the steroidogenic enzymes involved in ITT biosynthesis [37], acting on the negative feedback to reduce the levels of FSH and luteinizing hormone, thereby reducing testosterone levels [38], and by down-regulating expression of androgen receptor [39]. It is thus possible that genistein treatment suppresses ITT levels and stimulates spermatogenesis in rats treated with a chemotherapy drug.
The mechanism underlying the favorable suppression of ITT levels thus stimulating spermatogenesis in rat cytotoxic-drug-damaged testes has yet to be established. One potential mechanism whereby high levels of ITT may be detrimental to spermatogenesis is a change in the pattern of stem cell factor (SCF) expression [10]. It has been suggested that suppression of ITT would induce Sertoli cells to express more membrane-bound SCFs, which are essential for spermatogenesis. We plan to study the effect of genistein on SCF expression.
It is expected that practical hormonal treatment regimens will be developed that could help patients to recover fertility after chemotherapy. However, there may be species differences in the testicular responses to radiation and chemotherapy, GnRH analogs, or both, so that rescue protocols that are shown to be successful in rodents might not work in primates [40,41].
In conclusion, we observed that genistein effectively suppressed ITT levels and stimulated the recovery of spermatogenesis in rats treated with a chemotherapeutic drug. Genistein is a phytoestrogen that is more natural and has fewer side effects than estrogen hormone. This makes genistein a candidate substitute for GnRH analogs and estrogens, for helping to recover fertility after cancer therapy in humans. Further study is required to identify whether there are the species differences in the effects of genistein.
References
1. Kangasniemi M, Wilson G, Huhtaniemi I, Meistrich ML. Protection against procarbazine-induced testicular damage by GnRH-agonist and antiandrogen treatment in the rat. Endocrinology 1995;136:3677-3680.PMID: 7628410.


2. Meistrich ML, Kangasniemi M. Hormone treatment after irradiation stimulates recovery of rat spermatogenesis from surviving spermatogonia. J Androl 1997;18:80-87.PMID: 9089071.


3. Meistrich ML. Restoration of spermatogenesis by hormone treatment after cytotoxic therapy. Acta Paediatr Suppl 1999;88:19-22.PMID: 10626540.

4. Kangasniemi M, Wilson G, Parchuri N, Huhtaniemi I, Meistrich ML. Rapid protection of rat spermatogenic stem cells against procarbazine by treatment with a gonadotropin-releasing hormone antagonist (Nal-Glu) and an antiandrogen (flutamide). Endocrinology 1995;136:2881-2888.PMID: 7789313.


5. Shetty G, Wilson G, Huhtaniemi I, Shuttlesworth GA, Reissmann T, Meistrich ML. Gonadotropin-releasing hormone analogs stimulate and testosterone inhibits the recovery of spermatogenesis in irradiated rats. Endocrinology 2000;141:1735-1745.PMID: 10803584.


6. Shuttlesworth GA, de Rooij DG, Huhtaniemi I, Reissmann T, Russell LD, Shetty G, et al. Enhancement of a spermatogonial proliferation and differentiation in irradiated rats by GnRH antagonist administration. Endocrinology 2000;141:37-49.PMID: 10614621.


7. Ward JA, Robinson J, Furr BJA, Shalet SM, Morris ID. Protection of spermatogenesis in rats from the cytotoxic procarbazine by the depot formulation of Zoladex, a gonadotropin-releasing hormone agonist. Cancer spermatogenesis in rats from the cytotoxic procarbazine by the depot formulation of Zoladex, a gonadotropin-releasing hormone agonist. Cancer Res 1990;50:568-574.PMID: 2137024.

8. Meistrich ML, Wilson G, Kangasniemi M, Huhtaniemi I. Mechanism of protection of rat spermatogenesis by hormonal pretreatment: stimulation of spermatogonial differentiation after irradiation. J Androl 2000;21:464-469.PMID: 10819455.


9. Udagawa K, Ogawa T, Watanabe T, Yumura Y, Takeda M, Hosaka M. GnRH analog, leuprorelin acetate, promotes regeneration of rat spermatogenesis after severe chemical damage. Int J Urol 2001;8:615-622.PMID: 11903688.


10. Blanchard KT, Lee J, Boekelheide K. Leuprolide, a gonadotropin-releasing hormone agonist, reestablishes spermatogenesis after 2,5-hexanedione-induced irreversible testicular injury in the rat, resulting in normalized stem cell factor expression. Endocrinology 1998;139:236-244.PMID: 9421420.


11. Schoenfeld HA, Hall SJ, Boekelheide K. Continuously proliferative stem germ cells partially repopulate the aged, atrophic rat testis after gonadotropin-releasing hormone agonist therapy. Biol Reprod 2001;64:1273-1282.PMID: 11259276.


12. Udagawa K, Takeda M, Hosaka M, Kubota Y, Ogawa T. Recovery of spermatogenesis by high dose gonadotropin-releasing hormone analogue treatment in rat cryptorchid testis after orchiopexy. J Urol 2002;168:1279-1283.PMID: 12187282.


13. Meistrich ML, Shetty G. Inhibition of spermatogonial differentiation by testosterone. J Androl 2003;24:135-148.PMID: 12634296.


14. Meistrich ML, Wilson G, Shuttlesworth G, Huhtaniemi I, Reissmann T. GnRH agonists and antagonists stimulate recovery of fertility in irradiated LBNF1 rats. J Androl 2001;22:809-817.PMID: 11545294.


15. Shetty G, Weng CCY, Bolden-Tiller OU, Huhtaniemi I, Handelsman DJ, Meistrich ML. Effects of medroxyprogesterone and estradiol on the recovery of spermatogenesis in irradiated rats. Endocrinology 2004;145:4461-4469.PMID: 15205377.


16. Henriksson P, Carlstrom K, Pousette A, Gunnarsson PO, Johansson CJ, Eriksson B, et al. Time for revival of estrogens in the treatment of advanced prostatic carcinoma? Pharmacokinetics, and endocrine and clinical effects, of a parenteral estrogen regimen. Prostate 1999;40:76-82.PMID: 10386467.


17. Adlercreutz H, Mazur W. Phyto-estrogens and Western diets. Ann Med 1997;29:95-120.PMID: 9187225.


18. Kuiper GGJM, Carlsson B, Grandien K, Enmark E, Haggbald J, Nilsson S, et al. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors ╬▒ and ╬▓. Endocrinology 1997;138:863-870.PMID: 9048584.


19. Setchell KD, Cassidy A. Dietary isoflavones - biological effects and relevance to human health. J Nutr 1999;129:758S-767S.PMID: 10082786.


20. Trock BJ, Hilakivi-Clarke L, Clarke R. Meta-analysis of soy intake and breast cancer risk. J Natl Cancer Inst 2006;98:459-471.PMID: 16595782.


21. Akingbemi BT, Braden TD, Kemppainen BW, Hancock KD, Sherrill JD, Cook SJ, et al. Exposure to phytoestrogens in the perinatal period affects androgen secretion by testicular Leydig cells in the adult rat. Endocrinology 2007;148:4475-4488.PMID: 17569756.


22. Shao ZM, Wu J, Shen ZZ, Barsky SH. Genistein exerts multiple suppressive effects on human breast carcinoma cells. Cancer Res 1998;58:4851-4857.PMID: 9809990.

23. Valachovicova T, Slivova V, Bergman H, Shuherk J, Sliva D. Soy isoflavones suppress invasiveness of breast cancer cells by the inhibition of NF-kappaB/AP-1-dependent and -independent pathways. Int J Oncol 2004;25:1389-1395.PMID: 15492830.

24. Weber KS, Setchell KDR, Stocco DM, Lephart ED. Dietary soy-phytoestrogens decrease testosterone levels and prostate weight without altering LH, prostate 5alpha-reductase or testicular steroidogenic acute regulatory peptide levels in adult male Sprague-Dawley rats. J Endocrinol 2001;170:591-599.PMID: 11524239.


25. Skogseth H, Larsson E, Halgunset J. Inhibitors of tyrosine kinase inhibit the production of urokinase plasminogen activator in human prostatic cancer cells. APMIS 2005;113:332-339.PMID: 16011659.


26. Hewitt AM, Singletary KW. Soy extract inhibits mammary adenocarcinoma growth in a syngeneic mouse model. Cancer Lett 2003;192:133-143.PMID: 12668277.


27. Su SJ, Yeh TM, Chuang WJ, Ho CL, Chang KL, Cheng HL, et al. The novel targets for anti-angiogenesis of genistein on human cancer cells. Biochem Pharmacol 2005;69:307-318.PMID: 15627483.


28. Farina HG, Pomies M, Alonso DF, Gomez DE. Antitumor and antiangiogenic activity of soy isoflavone genistein in mouse models of melanoma and breast cancer. Oncol Rep 2006;16:885-891.PMID: 16969510.


29. McClive PJ, Sinclair AH. Type II and type IX collagen transcript isoforms are expressed during mouse testis development. Biol Reprod 2003;68:1742-1747.PMID: 12606408.


30. Anjamrooz SH, Movahedin M, Mowla SJ, Bairanvand SP. Assessment of morphological and functional changes in the mouse testis and epididymal sperms following busulfan treatment. Iran Biomed J 2007;11:15-22.PMID: 18051700.

31. Karashima T, Zalatnai A, Schally AV. Protective effects of analogs of luteinizing hormone releasing hormone against chemotherapy-induced testicular damage in rats. Proc Natl Acad Sci USA 1988;85:2329-2333.PMID: 2965391.



32. Kanatsu-Shinohara M, Toyokuni S, Morimoto T, Matsui S, Honjo T, Shinohara T. Functional assessment of self-renewal activity of male germline stem cells following cytotoxic damage and serial transplantation. Biol Reprod 2003;68:1801-1807.PMID: 12606387.


33. Fouchecourt S, Lareyre JJ, Chaurand P, DaGue BB, Suzuki K, Ong DE, et al. Identification, immunolocalization, regulation, and postnatal development of the lipocalin EP17 (epididymal protein of 17 kilodaltons) in the mouse and rat epididymis. Endocrinology 2003;144:887-900.PMID: 12586765.


34. Adlercreutz H, Mazur W, Bartels P, Elomaa V, Watanabe S, Wahala K, et al. Phytoestrogens and prostate disease. J Nutr 2000;130:658S-659S.PMID: 10702603.


35. Shetty G, Wilson G, Hardy MP, Niu E, Huhtaniemi I, Meistrich ML. Inhibition of recovery of spermatogenesis in irradiated rats by different androgens. Endocrinology 2002;143:3385-3396.PMID: 12193551.


36. Porter KL, Shetty G, Meistrich ML. Testicular edema is associated with spermatogonial arrest in irradiated rats. Endocrinology 2006;147:1297-1305.PMID: 16306082.


37. Sakaue M, Ishimura R, Kurosawa S, Fukuzawa NH, Kurohmaru M, Hayashi Y, et al. Administration of estradiol-3-benzoate down-regulates the expression of testicular steroidogenic enzyme genes for testosterone production in the adult rat. J Vet Med Sci 2002;64:107-113.PMID: 11913545.


38. Hossaini A, Dalgaard M, Vinggaard AM, Pakarinen P, Larsen JJ. Male reproductive effects of octylphenol and estradiol in Fischer and Wistar rats. Reprod Toxicol 2003;17:607-615.PMID: 14555199.


39. Sharpe RM. Pathways of endocrine disruption during male sexual differentiation and masculinization. Best Pract Res Clin Endocrinol Metab 2006;20:91-110.PMID: 16522522.


40. Brennemann W, Brensing KA, Leipner N, Boldt I, Klingmuller D. Attempted protection of spermatogenesis from irradiation in patients with seminoma by D-tryptophan-6 luteinizing hormone releasing hormone. Clin Investig 1994;72:838-842.


41. Kamischke A, Kuhlmann M, Weinbauer GF, Luetjens M, Yeung CH, Kronholz HL, et al. Gonadal protection from radiation by GnRH antagonist or recombinant human FSH: a controlled trial in a male nonhuman primate (Macaca fascicularis). J Endocrinol 2003;179:183-194.PMID: 14596670.


Figure┬Ā1
Schematic representation of experimental design. One hundred-fifty rats were equally divided into 5 groups. A busulfan injection was performed at week 0, and then a single injection of a GnRH agonist or oral administration of genistein for 4 weeks was performed at week 3. The rats were sacrificed by CO2 asphyxiation at experimental week 13 for evaluation of intra-testicular testosterone levels and histological analysis of the testes.

Figure┬Ā2
Effect of genistein treatment on the weight of testes reduced in size by busulfan treatment. All of the animals were sacrificed by CO2 asphyxiation at experimental week 13. Both testes were freed of the tunica and weighed. a,b,cNumbers in each group with different superscripts indicate significant difference (p<0.05).
Figure┬Ā3
Classification of seminiferous tubules in the chemically impaired testis according to the number of germ cell layers present. The testes were fixed in Bouin's fixative, dehydrated, and then embedded in paraffin. Thereafter, 5 ┬Ąm-thick serial sections were prepared and stained with hematoxylin and eosin for histological assessment. Seminiferous tubule cross-sections were observed and then classified as follows: (A) recovery (star, at least five layers), (B) half recovery (star, three or four layers), (C) poor recovery (star, one or two layers), (D) atrophy (star, no germ cells). For each rat, approximately 300 seminiferous tubules were evaluated in this way (├Ś200).
Figure┬Ā4
Effect of genistein treatment on the recovery of seminiferous tubules treated with busulfan. The effect of genistein was investigated with the percentages of the classified seminiferous tubules such as recovery (at least 5 layers), half recovery (3 or 4 layers), poor recovery (1 or 2 layers), and atrophy (no germ cells). a,b,cNumbers in each group with different superscripts indicate significant difference (p<0.05).
Figure┬Ā5
Effect of genistein treatment on intra-testicular testosterone (ITT) levels (ng/g) in rats treated with busulfan. One testis was homogenized and the level of ITT in the testicular supernatant in the homogenate was assayed at experimental week 13 by using T-antiserum-coated tubes. a,b,cNumbers in each groups with different superscripts indicate significant difference (p<0.05).
-
METRICS

- Related articles in Clin Exp Reprod Med
-
Effect of 19-Norandrostenedione on the Spermatogenesis in Rat Testis1990 ;17(1)
Effect of platelet activating factor on the secretion of progesterone in the rabbit.1992 June;19(1)
Effect of Leptin on the Steroidogenesis of Cultured Human Granulosa Cells.2000 March;27(1)





