Therapeutic effect of Ferula assa-foetida oleo-gum resin in rats with letrozole-induced polycystic ovary syndrome
Article information
Abstract
Objective
Asafoetida is a gum derived from Ferula assa-foetida, which is used in traditional Iranian medicine to treat some reproductive system disorders. The effects of asafoetida on ovarian tissue, expression of certain genes associated with polycystic ovary syndrome (PCOS), and levels of liver, kidney, and blood cell factors after treatment in a rat model were investigated.
Methods
Thirty rats were divided into five groups: normal, polycystic, and treatment with three doses of asafoetida (12.5, 25, and 50 mg/kg for 3 weeks after PCOS induction). PCOS was induced by letrozole at a dose of 1 mg/kg administered orally for 3 weeks. Blood samples were taken, and the ovaries were removed and prepared for histomorphometric examination. Liver and kidney parameters were measured. The mRNA expression levels of luteinizing hormone receptor, CYP11A1, adenosine monophosphate-activated protein kinase, adiponectin, and adiponectin receptors 1 and 2 were also measured by real-time polymerase chain reaction.
Results
The levels of liver, kidney, and blood parameters did not significantly differ between the treatment groups and the control group. At doses of 25 and 50 mg/kg, ovarian histopathology, especially the thicknesses of the theca and granulosa layers, was significantly improved relative to the PCOS group. The expression of target genes also improved in the 25 and 50 mg/kg treatment groups.
Conclusion
Asafoetida can be used to treat PCOS as a complementary approach to conventional therapies. Asafoetida appears to act by regulating and activating metabolic and ovarian cycle enzymes.
Introduction
Polycystic ovary syndrome (PCOS) is a common endocrine disorder among women that has adverse effects on childbearing. It can be associated with other diseases, including insulin resistance, diabetes mellitus, hyperlipidemia, and cardiovascular disease [1]. Common symptoms of PCOS include obesity, acne, irregular menstrual cycles, and infertility. Other symptoms such as anovulation, high androgen levels, and the presence of numerous ovarian cysts can also be seen [2]. High androgen levels are observed in approximately three-quarters of patients with PCOS. Hyperandrogenism is thought to be dependent on genetic factors and may also be influenced by environmental factors that impact the hypothalamic-pituitary-ovarian axis [3]. In animal models, letrozole is used to induce PCOS, and follicular atresia and abnormal follicular development have been observed in these models [4]. Several treatments are recommended for PCOS, such as lifestyle modification, pharmacotherapy, and herbal remedies [5]. Nowadays, attention is focused on the efficacy of plants used in traditional medicine because they are inexpensive and have minimal side effects [6]. The genus Ferula, belonging to the family of Apiaceae (Umbelliferae), consists of 170 species distributed worldwide. Of the 30 species of Ferula that have been found in Iran, 16 are native, and many are used in Iranian traditional medicine [7]. The major constituents of Ferula plants include sulfur-containing derivatives, coumarins, coumarin esters, sesquiterpenes, sesquiterpene lactones, sesquiterpene coumarins, glucuronic acid, galactose, arabinose, rhamnose, and daucane esters [8,9]. Ferula assa-foetida, a well-known species of Ferula, has traditionally been used for the treatment of various diseases including gastrointestinal disorders, nervous problems, and some reproductive system disorders, such as decreased libido [10]. In Iranian traditional medicine, Ferula assa-foetida oleo-gum resin (asafoetida) has been used as a menstrual enhancer. Also, in some regions of Iran, women with gynecological disorders such as dysmenorrhea [6] and oligomenorrhea [7] use Ferula assa-foetida as a remedy. The use of asafoetida during pregnancy has been banned due to its risk of induction of abortion. Modern investigations have shown that asafoetida has antifungal [11], antidiabetic [12], anti-inflammatory [13], antimutagenic [14], anticancer [15], antidementia [16], anticonvulsant [17], and antiviral [18] activities and also has a preventive effect against cuprizone-induced demyelination [19]. In a study on the effect of asafoetida on induced PCOS in rats, asafoetida resin extract was shown to increase the serum concentration of follicle-stimulating hormone and to significantly decrease concentrations of luteinizing hormone and testosterone in the treatment groups [20]. Additionally, Ghavi et al. [21] showed that the number of ovarian follicles and ovarian volume were significantly lower in women treated with asafoetida than in the control group. Based on the evidence and the lack of sufficient experimental studies, we chose to investigate the therapeutic effect of asafoetida on letrozole-induced PCOS in rats.
Methods
1. Animals
Thirty female Wistar rats (200–250 g) were obtained from the animal house of the Faculty of Medicine at Shahid Sadoughi University of Medical Sciences (Yazd, Iran) and kept under a standard cycle of 12 hours of light: 12 hours of darkness. Standard food was fed with water ad libitum. Experiments were performed in accordance with the recommendations of the University Ethics Committee of Laboratory Animals of Shahid Sadoughi University of Medical Sciences with approval ID IR.SSU.AEC.1401.005 on 2022-03-09. One week after the animals adapted to the environment, the experiments were initiated. To induce PCOS, letrozole (1 mg/kg) was given orally every day for 3 weeks [22]. To ensure induction of PCOS, the estrous cycle was assessed daily by vaginal smear with a light microscope to examine the ratio of leukocytes, epithelial cells, and cornified cells. The estrous cycle of rats usually lasts approximately 4 days in both control and PCOS animals, and rats that were in the estrous phase and had more cornified cells in the vaginal smear were selected for the study [23]. Asafoetida at concentrations of 12.5, 25, and 50 mg/kg was given orally every day for 2 weeks.
2. Preparation of plant oleo-gum resin
Ferula assa-foetida oleo-gum resin was collected from a mountainous area of Tabas (South Khorasan Province, Iran), and the plant species was identified and approved by Dr. Abbas Zarezadeh at the Yazd Agricultural Research Center. After drying in ambient air, the gum was soaked overnight in distilled water at room temperature, and the suspension was used orally. The concentration and dose of the suspension were expressed as the crude amount of dried oleo gum used in the preparation of the stock solution [24].
3. Biochemical, hematological, and hormonal analysis
Serum was prepared by collecting blood from the orbital sinuses of rats by centrifugation (3,000 rpm, 20 minutes). The serum was kept frozen until the biochemical assay. Levels of lactate dehydrogenase (LDH), aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), urea, and creatinine were measured using appropriate kits according to the manufacturers’ instructions. To count blood cells, sufficient blood was prepared, mixed with EDTA, and counted with a cell counter device. Serum testosterone levels were measured using a testosterone enzyme-linked immunosorbent assay kit (Abcam, Cambridge, UK).
4. Histopathological analysis
Ovaries of the rats were removed prior to histological examination. Ovarian tissues were fixed in 10% neutral formalin. Then, all samples were cleaned, dehydrated, and embedded in paraffin. A microtome (Leica, Wetzlar, Germany) was used to prepare 7-μm-thick sections, and the slides were stained with hematoxylin and eosin dye. The ovarian morphology and histopathological changes were observed microscopically. Follicles were grouped according to the following definitions: (1) Preantral follicle: a follicle with an intact, enlarged oocyte with a visible nucleus and a single layer of cuboidal granulosa cells. (2) Antral follicle: a follicle with two or more layers of cuboidal granulosa cells, regardless of whether the cavity is apparent. (3) Atretic follicle: a follicle containing a degenerating ovum or pyknotic granulosa cells. (4) Cystic follicle: a follicle appearing as a large fluid-filled structure with an attenuated granulosa cell layer and a thickened theca internal cell layer. For each group, the number of corpus luteum and preantral, antral, atretic, and cystic follicles of the ovaries were evaluated. The thicknesses of the theca interna cells and granulosa layer of the oocyte were evaluated. The layer thickness was measured using Micrometrics SE Premium Software (Accu-Scope Inc., Commack, NY, USA).
5. Reverse transcription and real-time polymerase chain reaction
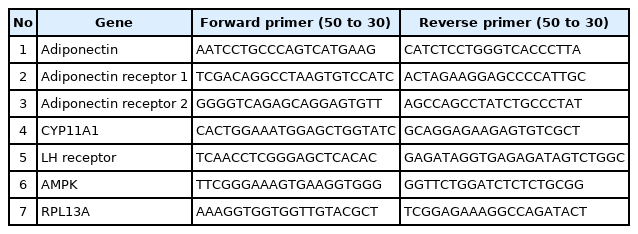
Total RNA was extracted with RNX-Plus solution (Sinaclon, Tehran, Iran) at the Central Research Laboratory of Shahid Sadoughi University of Medical Sciences in Yazd, Iran in accordance with the manufacturer’s instructions. Complementary DNA (cDNA) was synthesized from 1 μg of total RNA using a Thermo Scientific RevertAid First Strand cDNA Synthesis Kit (Parstous, Mashhad, Iran). The cDNA was amplified via reverse-transcription polymerase chain reaction (PCR) using AmpliTaq Gold DNA polymerase and quantitative real-time PCR and by using Premix Ex Taq mix (Yekta Tajhiz, Tehran, Iran) with SYBR Green I dye (Molecular Probes, Eugene, OR, USA) in a Step One Plus system (Applied Biosystems, Waltham, MA, USA). The primers used for reverse-transcription PCR were synthesized by Betagen Inc. (Mashhad, Iran) and are shown in Table 1. All experiments were performed in duplicate, and messenger RNA values were calculated based on the cycle threshold and monitored to obtain an amplification curve.
6. Statistical analysis
The results are reported as the mean±standard error of the mean. Differences between means were obtained using one-way analysis of variance (Tukey-Kramer method) performed with Graph Pad Prism version 8 software (GraphPad Inc., San Diego, CA, USA).
Results
1. Effect of asafoetida on body weight gain
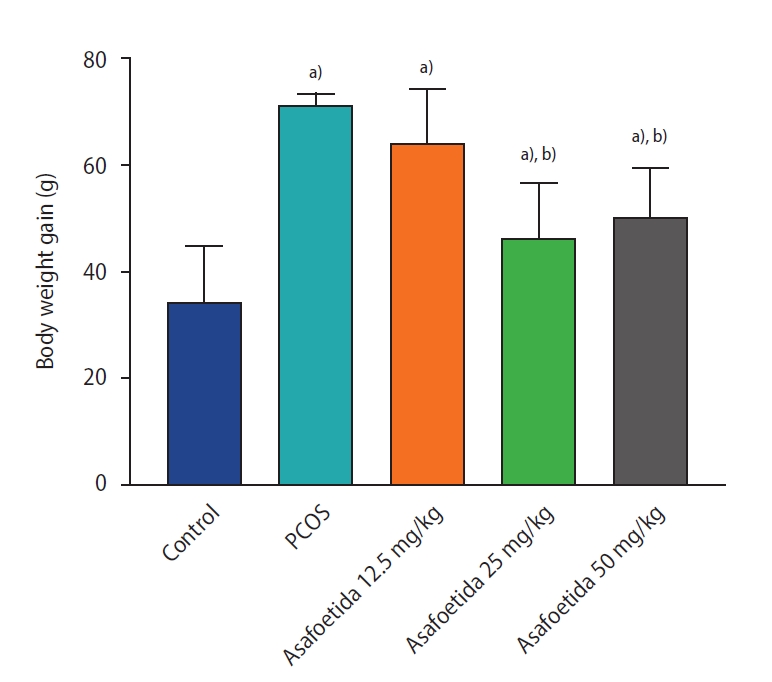
In the present study, a larger amount of body weight gain was observed in the PCOS group than in control rats, but the treatment of PCOS rats with 25 and 50 mg/kg of asafoetida resulted in less weight gain relative to the PCOS group (p<0.05) (Figure 1).
2. Biochemical, hematological, and hormonal analysis
Serum biochemical (including urea, creatinine, AST, ALT, ALP, and LDH) and hematological parameters are summarized in Tables 2 and 3. No significant change was observed at any concentration of asafoetida relative to the control group. Serum testosterone levels in the groups treated with 25 and 50 mg/kg asafoetida were significantly lower than in the PCOS group.
3. Histopathological results
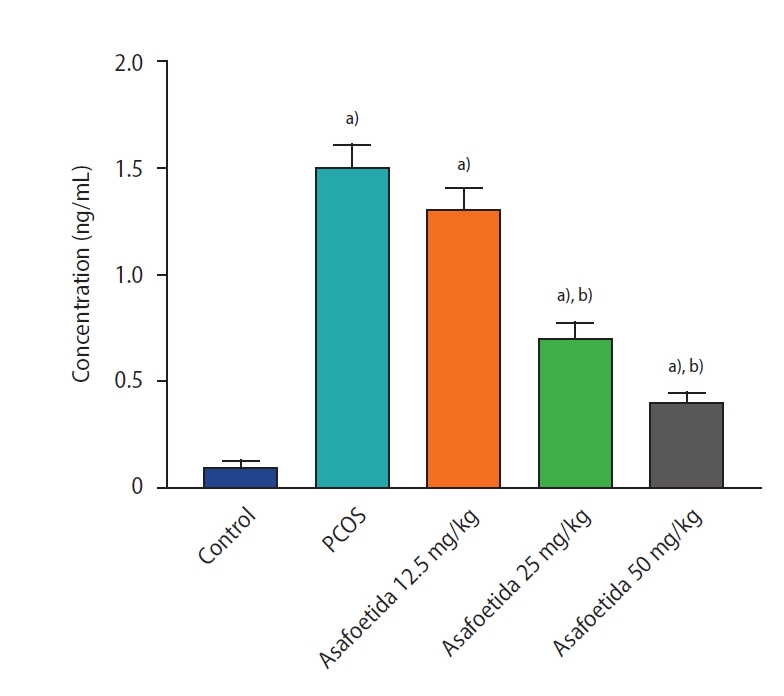
Histopathological examination of ovaries from the control group revealed numerous ovarian follicles at various grades of maturation surrounded by dense ovarian stroma (Figure 2A). Follicular cysts were observable on the ovaries of the PCOS group as fluid-filled sacs, and histological examination revealed large cystic follicles filled with granulosa cells and surrounded by dense ovarian stroma. PCOS rats exhibited cystic, atretic, and antral follicles and fewer corpora lutea (Figure 2B). In the groups treated with 25 and 50 mg/kg of asafoetida, increases in the numbers of antral follicles and corpora lutea were observed in the ovarian sections (Figure 2C-E). An increase in the thickness of the theca layer in the cystic follicles was seen in the PCOS group compared with the antral follicles of the control group, and both 25 and 50 mg/kg of asafoetida mitigated this effect significantly (p<0.05) (Figure 3). Plasma testosterone levels were significantly lower in the asafoetida-treated groups than in the PCOS group (p<0.05) (Figure 4).
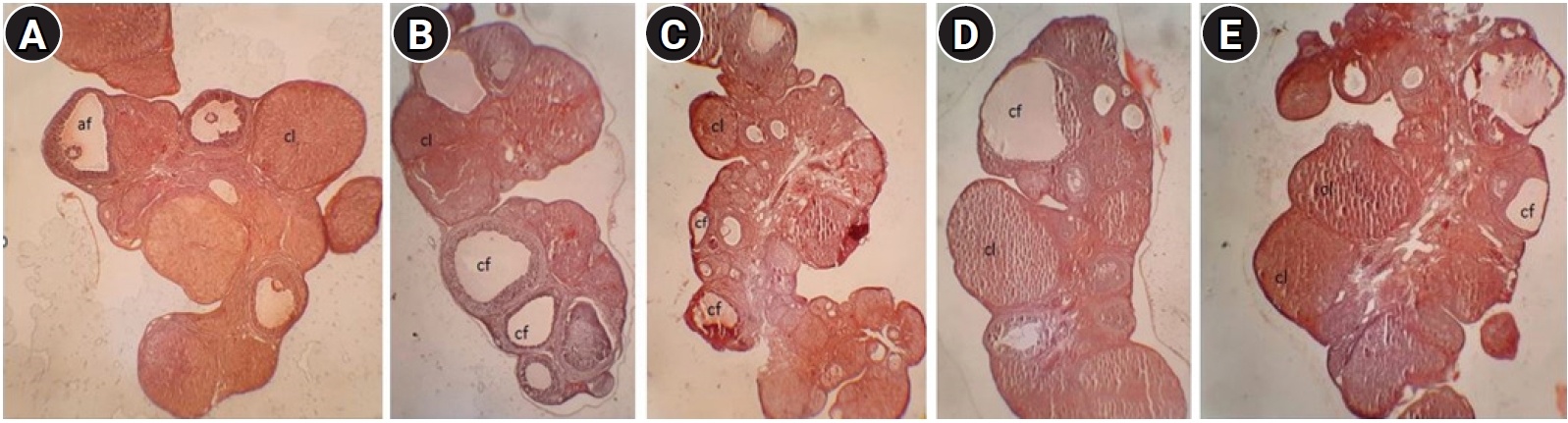
Photomicrograph of ovarian tissue from different groups. (A) Control group: section of ovary from a control rat showing follicles at various stages. (B) Polycystic ovary syndrome (PCOS) group: section of ovary from a PCOS rat showing cystic follicles (cf), antral follicles, and a decreased number of corpora lutea (cl). (C-E) Asafoetida group: the ovarian sections indicate a decrease in numbers of atretic and cystic follicles with increases in the number of antral follicles (af) and corpora lutea size (H&E, ×200).
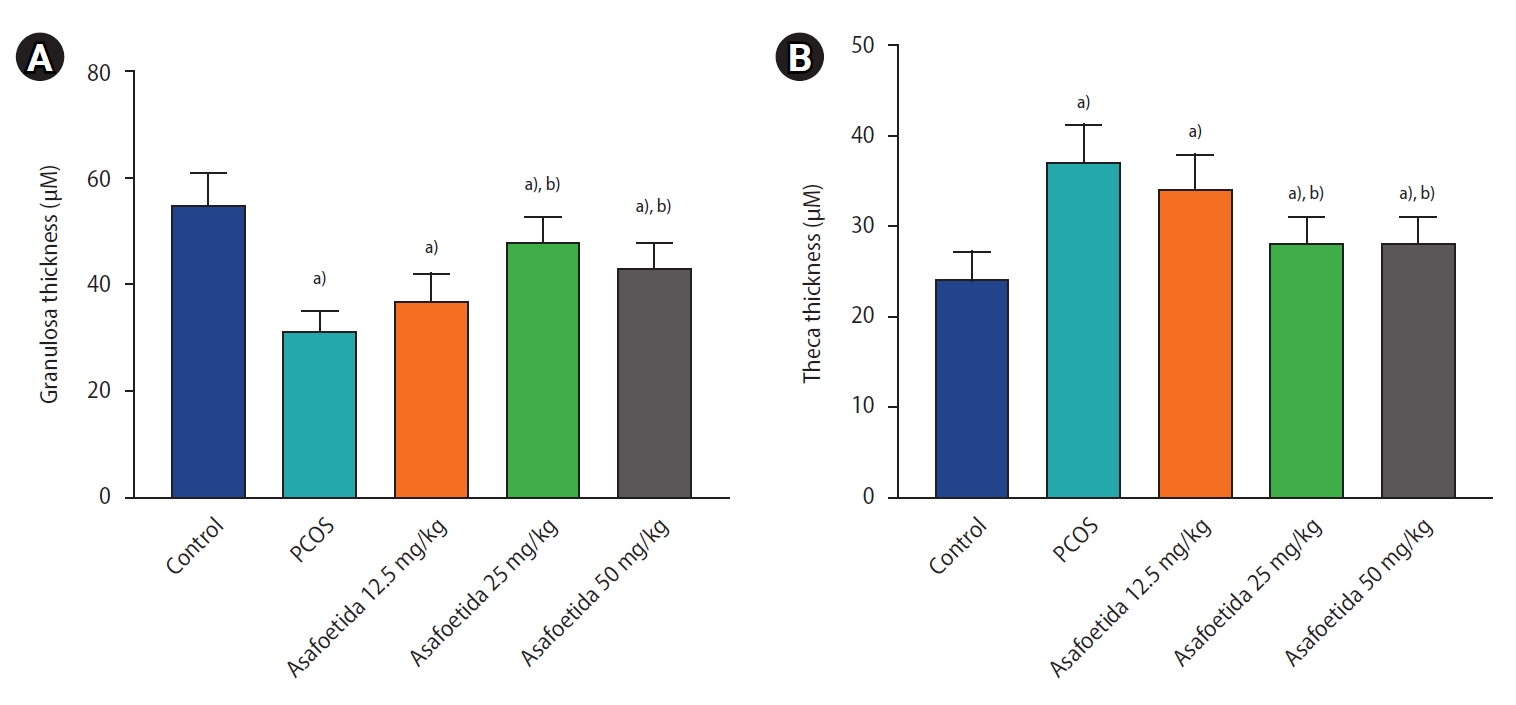
Comparison of morphometrical parameters. The thickness of the granulosa layer (A) and the theca interna cells (B) of the oocyte by group. Values are presented as mean±standard error of the mean (n=5). a)p<0.05, vs. control group; b)p<0.05, vs. polycystic ovary syndrome (PCOS) group.
4. Effect of asafoetida on gene expression
The effects of asafoetida on the mRNA expression of luteinizing hormone receptor (LHR), CYP11A1, adenosine monophosphate-activated protein kinase (AMPK), adiponectin, and adiponectin receptor 1 and 2 in ovarian tissue were also determined. Expression levels of LHR, AMPK, adiponectin, and adiponectin receptor 1 and 2 were significantly lower in the untreated PCOS group compared to the control group. However, in the groups treated with 25 and 50 mg/kg of asafoetida, the expression levels of these genes were significantly greater than in the PCOS group. The expression of the CYP11A1 gene was significantly elevated in the PCOS group relative to the control and lowered in the groups treated with 25 and 50 mg/kg of asafoetida relative to the PCOS group (p<0.05) (Figure 5).
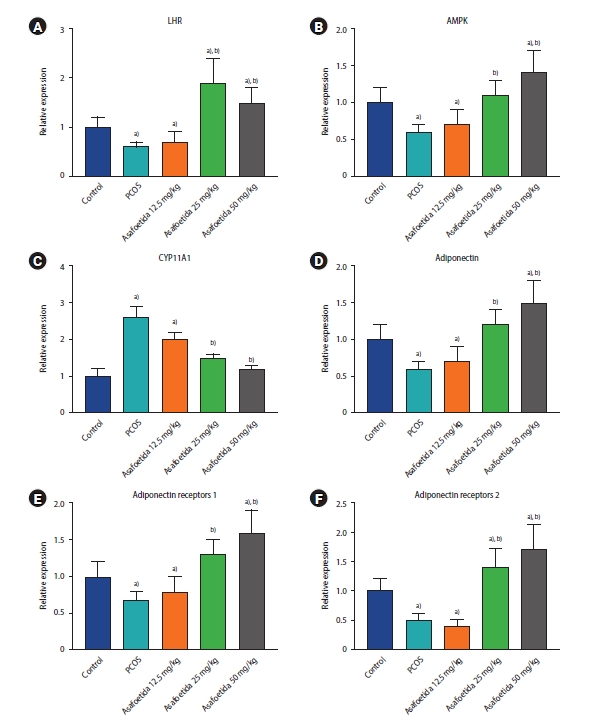
Effects of asafoetida on the gene expression of luteinizing hormone receptor (LHR; A), adenosine monophosphate-activated protein kinase (AMPK; B), CYP11A1 (C), adiponectin (D), and adiponectin receptors 1 and 2 (E, F) using real-time polymerase chain reaction. Values are presented as mean±standard error of the mean (n=5). a)p<0.05, vs. control group; b)p<0.05, vs. polycystic ovary syndrome (PCOS) group.
Discussion
PCOS is a metabolic disorder related to the disruption of endocrine function regulation. This disorder is characterized by symptoms such as irregular ovulation, abdominal obesity, and hyperandrogenism [25]. Our study showed that PCOS can cause significant weight gain, and asafoetida in doses of 25 and 50 mg/kg decreases the amount of that gain. Weight gain is one of the metabolic changes associated with PCOS. In a previous study, rats with letrozole-induced PCOS exhibited increased body weight, just like women with PCOS [22]. The results of the present biochemical and hematological analysis indicated that asafoetida did not cause toxic effects in the animals. Although few studies exist regarding the toxic effects of asafoetida, Iranian traditional medicine emphasizes that high doses of asafoetida can lead to swollen lips, gastrointestinal disorders such as bloating and diarrhea, discomfort, and headaches [10]. Bagheri et al. [26] reported that asafoetida had no acute toxic effects, but long-term use, especially at high doses, may damage the liver, kidneys, or bone marrow tissue. Additionally, oral consumption of Ferula assa-foetida extract in rats for 28 consecutive days was found to have no significant impact on body weight, blood parameters, and activity levels of AST, ALT, ALP, creatinine, and urea [27]. The histopathological results of the present study showed that asafoetida improved the morphology of the ovarian tissue in rats with PCOS. Follicular dysfunction, such as the presence of large atretic cysts with letrozole-induced small granulosa cells, has been observed in previous studies [28]. In the current study, treatment with asafoetida, especially at doses of 25 and 50 mg/kg, restored the components and morphology of the ovarian follicles to normal. This indicates that the extract is involved in regulating various factors associated with follicular growth in the ovary. A study by Ghavi et al. [21], conducted with 34 women, showed that receiving 100 mg of asafoetida for 2 months decreased the number of ovarian follicles in women with PCOS. An increase in testosterone level, which causes acne and hair in women with PCOS, is a key challenge in this disease. Elevated testosterone levels have been shown to contribute to the pathogenesis of PCOS, and suppression of these high testosterone concentrations may have beneficial effects in individuals with PCOS disorders [29]. Bagheri et al. [30] found that asafoetida could increase sperm count in male rats after 6 weeks of low-dose treatment, but high doses had detrimental effects on Leydig cells and were associated with decreased testosterone concentration. Another study similarly showed that injection of 75, 150, and 300 mg/kg of asafoetida extract for 14 days thickened the seminiferous layers and decreased the number of Leydig cells and testosterone concentration at a dose of 300 mg/kg [31]. Morovatisharifabad et al. [20] also showed that testosterone levels decreased significantly in PCOS rats treated with hydroalcoholic extract of asafoetida gum. LHR gene expression can be an indication of the capacity of drug compounds to treat PCOS. LHR is found on theca and granulosa cell surfaces, and LHR transcript levels are associated with ovulation, corpus luteum formation, and the production of steroids, including estrogen, progesterone, and androgens [32]. In the present study, LHR transcription levels were altered in the ovaries of the PCOS group, and these levels returned to normal with asafoetida treatment. Additionally, the mRNA expression level of CYP11A1, which encodes a key enzyme in the synthesis of steroid hormones, was significantly increased in the PCOS group, a trend that was mitigated by treatment with asafoetida. Expression levels of AMPK, adiponectin, and adiponectin receptor 1 and 2 were lower in the PCOS group than the control group, while treatment with asafoetida increased the expression of all of these and may thus restore a regular menstrual cycle. AMPK is a regulator of energy homeostasis that facilitates coordination in metabolic pathways and is a potential therapeutic target in metabolic disorders [33]. In contrast, adiponectin is a hormone that is released from fat tissue, and its levels are inversely related to fat mass [34]. Various animal studies have shown that the expression levels of adiponectin and AMPK are significantly reduced in animals with PCOS, while this effect is reversed in groups with effective treatment [35]. The expression of the adiponectin gene would be expected to increase in the treatment groups. The results of our study are consistent with this evidence, and the expression levels of AMPK, adiponectin, and adiponectin receptors were significantly elevated in the groups treated with asafoetida compared to the PCOS control group. While the mechanism of action of asafoetida in the treatment of PCOS cannot be said with certainty, some evidence may be helpful in clarifying the issue. The potential effect of asafoetida in fat metabolism has been studied, and taking asafoetida has been shown to significantly reduce body weight and abdominal fat at doses of 25 and 50 mg/kg, an effect that is associated with decreased serum leptin levels [36]. Therefore, the effect of asafoetida on PCOS may be related to energy metabolism [37]. Based on the present findings, asafoetida may exert its protective effects on PCOS rats via the AMPK and adiponectin pathways and, by countering metabolic disorders, aid in PCOS treatment.
The results of the present study suggest that asafoetida may be a potential treatment for hormonal disorders associated with PCOS. These findings may be due to interactions with the hypothalamic-pituitary-ovarian axis or effects on metabolic processes. Despite this, based on the obtained data, the effects of asafoetida on PCOS cannot be clarified for certain, and some aspects are still unclear. Since asafoetida contains different compounds, more studies should be carried out on its different fractions to isolate its active compounds. Also, by conducting controlled studies on humans, the effects of this herbal product in the treatment of PCOS can be better understood.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Author contributions
Conceptualization: SMB. Data curation: AS, MY. Formal analysis: SMB. Funding acquisition: AS. Methodology: DJ. Project administration: SMB. Visualization: ZF. Writing–original draft: SMB, ZF. Writing–review & editing: MY.