|
 |
- Search
| Clin Exp Reprod Med > Volume 40(1); 2013 > Article |
Abstract
Objective
The aim of the present study was to examine the relationship among male age, strict morphology, and sperm chromatin structure and condensation.
Methods
Sperm samples from a total of 100 men underwent semen analysis, and sperm chromatin structure and condensation were assessed with toluidine blue (TB) and aniline blue (AB) tests.
Results
Prevalence of strict morphology of less than 4%, and abnormal sperm chromatin structure and condensation did not show any statistically significant differences according to male age (p=0.605, p=0.235, and p=0.080). No significant correlation was demonstrated among age of male partners, strict morphology, and abnormal sperm chromatin structure using TB and AB tests. However, abnormal sperm chromatin condensation was positively associated with sperm chromatin structure (r=0.594, p=0.000) and showed negative correlation with strict morphology (r=-0.219, p=0.029).
Semen analysis has been used as the first step in the determination of male factor infertility and semen quality is determined according to the concentration, motility, and morphology of the spermatozoa. However, semen parameters set by the World Health Organization (WHO) have been criticized for inadequate discriminative power in the assessment of male infertility [1], and values for these standard semen parameters do not exclude the possibility of normal fertility [2].
Therefore, the development of new tests that differentiate between fertile and infertile men is needed. Recently, several studies have indicated an increase in the rates of sperm chromosomal aneuploidy, sperm DNA, and chromatin condensation abnormalities in semen samples of male partners from couples with recurrent spontaneous abortion (RSA) compared to fertile controls [3-6]. However, on the other hand, other studies have reported that sperm DNA integrity is not associated with unexplained RSA [7,8].
To detect these sperm abnormalities, several techniques including cytochemical assays, flow cytometic-based sperm chromatin structure assay, comet assay, and terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling (TUNEL) assay have been investigated. Cytochemical assays are sensitive, simple, and inexpensive since they do not require special instruments such as flow cytometry [6]. DNA single and double strand breaks appear in the fully mature sperm [9]. Toluidine blue (TB) staining has been reported to be a sensitive test for incomplete DNA structure and packaging [6]. Additionally, aniline blue (AB) staining is used for visualization of sperm chromatin condensation [10]. This staining is based on the detection of lysine residues with AB as a measure of an excess of histones remaining bound to the sperm DNA [11]. The chromosomes of sperm cells are tightly packaged into a complex of DNA and protamines, as somatic histones are replaced during spermiogenesis [12].
The aim of the present study was to examine the relationship among male age, strict morphology, sperm chromatin structure, and condensation evaluated by TB and AB tests. Moreover, we aimed to assess whether the routine use of these tests for male partners is useful.
A total of 100 semen samples were obtained from men visiting our laboratory for infertility evaluation. The average age of the males was 37.6 years. This study was approved by the Institutional Review Board of the Seoul National University Hospital (H-1012-102-345) and informed written consent was obtained from each participant.
After avoiding coitus for at least three days, all semen samples were obtained by masturbation at the time of semen analysis or oocyte pick-up. After liquefaction for 30 minutes at room temperature, each sample was routinely assessed using computer-assisted semen analysis (CASA, FAS2011, Medical Supply Co., Seoul, Korea). Semen quality was used to analyze the sperm parameters (volume, CASA, and strict morphology) according to the WHO criteria [1].
Thereafter, several smears were prepared from each specimen to record the strict morphology and chromatin status, using TB and AB staining. For the strict morphology, Hemacolor (Merck, Darmstadt, Germany) staining was done, and 200 spermatozoa were analyzed under light microscope using oil immersion with magnification of ×1,000. If the percentage of normal sperm was the same or greater than 4%, it was considered normal.
The TB stain was performed as described earlier [13,14]. Briefly, thin smears were prepared on silane-coated slides (MUTO Pure Chemicals Co. Ltd., Tokyo, Japan). Air-dried smears were fixed in freshly prepared 96% ethanol-acetone (1:1) at 4℃ for 1 hour and air dried, then hydrolyzed in 0.1 N HCl at 4℃ for 5 minutes. Thereafter, the slides were rinsed 3 times in distilled water for 2 minutes and finally stained with 0.05% TB (in 50% McIlvaine's citrate phosphate buffer, pH 3.5, Merck) for 5 minutes at room temperature. The slides were rinsed briefly in distilled water.
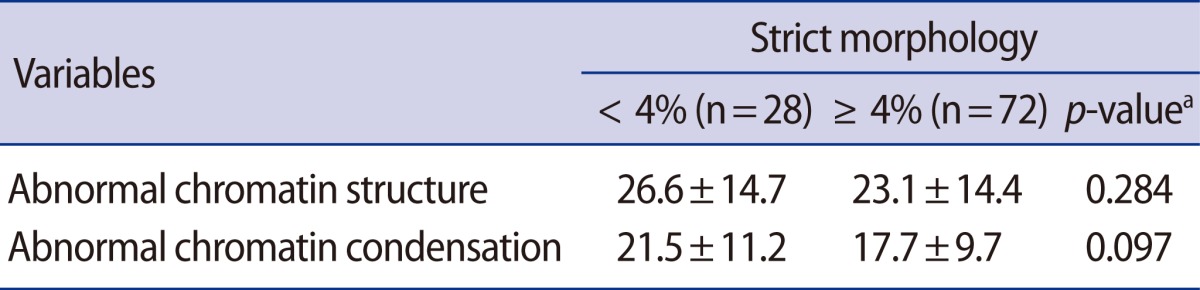
Under light microscopic evaluation, a total of 300 spermatozoa were counted in different areas of each slide using oil immersion with ×1,000 magnification. Sperm cell heads with good chromatin integrity were light blue; those of diminished integrity were deep violet (purple). Deep violet sperms were considered to be abnormal (Figure 1), and the percentage of sperms with a deep violet color was determined.
To perform this staining, sperms were stained with AB-eosin as described in a previous report [15,16]. The slides were prepared by smearing 10 µL of each raw semen sample. The slides were air-dried and then fixed in 4% formalin (Junsei Chemical Co., Ltd., Tokyo, Japan) for 5 minutes at room temperature, rinsed in water, and stained in 5% aniline blue (Sigma-Aldrich Co., St. Louis, MO, USA) in 4% acetic acid (pH 3.5) solution for 5 minutes. After 5 minutes, the slides were rinsed in water and stained in 0.5% eosin (Merck) for 1 minute. This was followed by rinsing and air drying of the slides. Under light microscopic evaluation, 300 spermatozoa were counted in different areas of each slide using ×1,000 magnification. Immature sperm were stained dark blue by the eosin counterstain. The percentage of abnormal sperm chromatin condensation was calculated as the ratio of the number of dark-blue sperm to the total number of sperm analyzed.
The study participants were grouped according to age as described by de la Rochebrochard and Thonneau [17]: ≤34, 35-39, and ≥40 years.
Statistical analysis was performed using SPSS ver. 19.0 (IBM, Armonk, NY, USA). Statistical tests including the Student's t-test, one-way analysis of variance, Pearson's correlation, and linear regression were performed, and p<0.05 was considered to be statistically significant.
The mean age of the study participants was 37.6±5.0, and the semen samples tested had a mean strict morphology of 5.9%±3.6%, a mean abnormal sperm structure of 24.1%±14.5%, and a mean abnormal sperm condensation of 18.8%±10.2%.
The percentage of abnormal sperm chromatin structure and condensation was compared between the two strict morphology groups (Table 1). No statistically significant differences were found between the normal and abnormal strict morphology groups (p>0.05).
Participants were classified by age into three groups as follows: group 1 of age ≤34 (n=32), group 2 aged between 35 and 39 years (n=34) and group 3 of age ≥40 years (n=34) (Table 2). The prevalence of abnormal strict morphology, and abnormal sperm chromatin structure and condensation did not show any statistically significant difference according to male age (p=0.605, p=0.235, and p=0.080).
Table 3 demonstrates Pearson's correlation coefficient among male age, strict morphology, abnormal sperm chromatin structure, and condensation. Although no significant correlations were observed among male age, strict morphology, and abnormal sperm chromatin structure (p>0.05), sperm chromatin condensation showed good correlation with strict morphology (r=-0.219, p=0.029) and a positive and significant correlation existed with sperm chromatin structure (r=0.594, p=0.000) (Figure 2).
Sperm chromatin condensation was negatively correlated with strict morphology in the overall strict morphology (r=-0.219, p=0.0029). This significant correlation, however, was lost when the strict morphology was split into the two groups according to the lower reference limit for normal forms (independent-samples t-test: p=0.097) (Figure 3).
The diagnosis of male infertility has been traditionally diagnosed based upon analysis of sperm concentration, motility, and morphology as routine indicators of human semen quality [18]. However, these parameters may not reveal subtle defects such as sperm DNA damage. Previous studies investigating sperm DNA damage have shown that sperm damage is more common in infertile men and also associated with spontaneous recurrent miscarriage [6]. Although the mechanisms of sperm chromatin abnormalities in human spermatozoa are not completely understood, defective sperm chromatin packaging, abortive apoptosis, and oxidative stress have been proposed [10]. Different methods have been suggested for evaluating sperm DNA damage. Since studies have not extensively investigated the relationship between sperm DNA damage and reproductive outcomes, the routine use of DNA integrity tests in the clinical evaluation of male factor infertility is controversial [19,20].
In the present study, the relationship between sperm abnormal chromatin structure and condensation was evaluated by cytochemical assays, and the clinical usefulness of these tests was also assessed.
Hammadeh et al. [21] carried out AB staining to determine the value of analysis of sperm chromatin condensation in the assessment of male fertility. They reported that a significant difference was observed between patients and healthy donors. However, no correlation was found between sperm chromatin condensation and morphology, count, and motility. Their results suggested that chromatin condensation constitutes a valuable parameter in the assessment of male fertility, completely independent of conventional sperm parameters. In our results, although the percentage of abnormal sperm chromatin structure and condensation between the normal and abnormal strict morphology groups did show no statistically significant differences (p>0.05), abnormal sperm chromatin condensation was negatively correlated with strict morphology in the overall strict morphology analysis (p<0.005). These results correspond well with those of the earlier study that reported that the percentage of normal sperm chromatin condensation heads was much higher in the population of morphologically normal forms than in that of abnormal forms [11].
This study also investigated the influence of male age on strict morphology, abnormal sperm chromatin structure, and condensation. The results did not show any statistical significance according to male age (p=0.605, p=0.235, and p=0.080). Nijs et al. [22] proposed that their data on 278 patients undergoing a first IVF or ICSI treatment could not demonstrate any male age-related influences on sperm concentration, motility, or morphology; neither was a significant increase in DNA fragmentation nor the presence of immature chromatin (measured sperm chromatin structural assay) noted. Wong et al. [15] compared the AB assay with and without eosin, and searched for a correlation between the results and pregnancy outcomes after the ICSI procedure. They suggested that adding eosin counterstain to AB improved assessment of chromatin condensation and found no correlation between chromatin condensation and ICSI fertilization or male age.
Although sperm DNA damage is associated with lower pregnancy and ongoing pregnancy rates, a clinically relevant standard DNA damage test with a meaningful cut-off level has not yet been reported [23]. In a systematic review on the predictive value of DNA integrity tests (SCSA and TUNEL), Collins et al. [7] reported that routine testing for sperm DNA integrity is clinically worthwhile for infertile couples undergoing IVF and ICSI treatment. They suggested that the results do not provide a clinical indication for routine use of these tests in male evaluation. However, Talebi et al. [6] examined the possible relationship between sperm DNA integrity and chromatin packaging evaluated by cytochemical assays (AB, chromomycin A3, toluidine blue, acridine orange), traditional sperm parameters, and RSA of unknown origin. Their data showed that the RSA group had less chromatin condensation and poorer DNA integrity than fertile men with no history of RSA, and that the strict morphology did not differ between the two groups.
In the present study, there are a few limitations. Firstly, the sample size of the study was small, and this might have limited the examination of clinical usefulness. Our findings could be due to chance and should be interpreted with caution. Second, the data did not include clinical outcomes including the fertilization or pregnancy rate.
In conclusion, the test for sperm chromatin condensation was significantly correlated with sperm morphology. If CASA and strict morphology are routinely used to assess male partners, it may be sufficient to recommend them to patients. However, sperm chromatin tests may be considered in the evaluation of selected couples, including those with RSA and unexplained infertility.
Notes
References
1. World Health Organization. WHO laboratory manual for the examination and processing of human semen [Internet]. c2013. cited 2013 Mar 4. 5th ed. Geneva: World Health Organization; Available from: http://www.who.int/reproductivehealth/publications/infertility/9789241547789/en/index.html
2. Guzick DS, Overstreet JW, Factor-Litvak P, Brazil CK, Nakajima ST, Coutifaris C, et al. Sperm morphology, motility, and concentration in fertile and infertile men. N Engl J Med 2001;345:1388-1393.PMID: 11794171.


3. Evenson DP, Jost LK, Marshall D, Zinaman MJ, Clegg E, Purvis K, et al. Utility of the sperm chromatin structure assay as a diagnostic and prognostic tool in the human fertility clinic. Hum Reprod 1999;14:1039-1049.PMID: 10221239.


4. Carrell DT, Liu L, Peterson CM, Jones KP, Hatasaka HH, Erickson L, et al. Sperm DNA fragmentation is increased in couples with unexplained recurrent pregnancy loss. Arch Androl 2003;49:49-55.PMID: 12647778.


5. Virro MR, Larson-Cook KL, Evenson DP. Sperm chromatin structure assay (SCSA) parameters are related to fertilization, blastocyst development, and ongoing pregnancy in in vitro fertilization and intracytoplasmic sperm injection cycles. Fertil Steril 2004;81:1289-1295.PMID: 15136092.


6. Talebi AR, Vahidi S, Aflatoonian A, Ghasemi N, Ghasemzadeh J, Firoozabadi RD, et al. Cytochemical evaluation of sperm chromatin and DNA integrity in couples with unexplained recurrent spontaneous abortions. Andrologia 2012;44(Suppl 1): 462-470.PMID: 21806662.


7. Collins JA, Barnhart KT, Schlegel PN. Do sperm DNA integrity tests predict pregnancy with in vitro fertilization? Fertil Steril 2008;89:823-831.PMID: 17644094.


8. Bellver J, Meseguer M, Muriel L, Garcia-Herrero S, Barreto MA, Garda AL, et al. Y chromosome microdeletions, sperm DNA fragmentation and sperm oxidative stress as causes of recurrent spontaneous abortion of unknown etiology. Hum Reprod 2010;25:1713-1721.PMID: 20501469.


9. Marcon L, Boissonneault G. Transient DNA strand breaks during mouse and human spermiogenesis new insights in stage specificity and link to chromatin remodeling. Biol Reprod 2004;70:910-918.PMID: 14645105.


10. Agarwal A, Erenpreiss J, Sharma R. In: Gardner DK, Weissman A, Howles CM, Shoham Z, editors. Sperm chromatin assessment. Textbook of assisted reproductive technologies. 2009. 3rd ed. London: Informa Healthcare; p. 67-84.

11. Dadoune JP, Mayaux MJ, Guihard-Moscato ML. Correlation between defects in chromatin condensation of human spermatozoa stained by aniline blue and semen characteristics. Andrologia 1988;20:211-217.PMID: 3177899.


12. Braun RE. Packaging paternal chromosomes with protamine. Nat Genet 2001;28:10-12.PMID: 11326265.


13. Erenpreiss J, Bars J, Lipatnikova V, Erenpreisa J, Zalkalns J. Comparative study of cytochemical tests for sperm chromatin integrity. J Androl 2001;22:45-53.PMID: 11191087.


14. Erenpreiss J, Jepson K, Giwercman A, Tsarev I, Erenpreisa J, Spano M. Toluidine blue cytometry test for sperm DNA conformation: comparison with the flow cytometric sperm chromatin structure and TUNEL assays. Hum Reprod 2004;19:2277-2282.PMID: 15271869.


15. Wong A, Chuan SS, Patton WC, Jacobson JD, Corselli J, Chan PJ. Addition of eosin to the aniline blue assay to enhance detection of immature sperm histones. Fertil Steril 2008;90:1999-2002.PMID: 18177870.


16. Park YS, Kim MK, Lee SH, Cho JW, Song IO, Seo JT. Efficacy of testicular sperm chromatin condensation assay using aniline blue-eosin staining in the IVF-ET cycle. Clin Exp Reprod Med 2011;38:142-147.PMID: 22384433.



17. de la Rochebrochard E, Thonneau P. Paternal age and maternal age are risk factors for miscarriage; results of a multicentre European study. Hum Reprod 2002;17:1649-1656.PMID: 12042293.


18. Barratt CL, Aitken RJ, Bjorndahl L, Carrell DT, de Boer P, Kvist U, et al. Sperm DNA: organization, protection and vulnerability: from basic science to clinical applications--a position report. Hum Reprod 2010;25:824-838.PMID: 20139429.


19. The Practice Committee of the American Society for Reproductive Medicine. The clinical utility of sperm DNA integrity testing. Fertil Steril 2008;90:S178-S180.PMID: 19007622.


20. The Practice Committee of the American Society for Reproductive Medicine. Diagnostic evaluation of the infertile male: a committee opinion. Fertil Steril 2012;98:294-301.PMID: 22698639.


21. Hammadeh ME, Zeginiadov T, Rosenbaum P, Georg T, Schmidt W, Strehler E. Predictive value of sperm chromatin condensation (aniline blue staining) in the assessment of male fertility. Arch Androl 2001;46:99-104.PMID: 11297072.


22. Nijs M, De Jonge C, Cox A, Janssen M, Bosmans E, Ombelet W. Correlation between male age, WHO sperm parameters, DNA fragmentation, chromatin packaging and outcome in assisted reproduction technology. Andrologia 2011;43:174-179.PMID: 21561463.


23. Beshay VE, Bukulmez O. Sperm DNA damage: how relevant is it clinically? Curr Opin Obstet Gynecol 2012;24:172-179.PMID: 22366964.


Figure 1
(A) Sperm chromatin structure assessed by toluidine blue staining. Sperm cell heads with good chromatin structure were light blue; those of abnormal chromatin structure were deep violet (arrow, ×1,000). (B) Sperm chromatin condensation assessed by aniline blue. Immature sperm stained dark blue by the eosin counterstain (arrowheads, ×1,000).
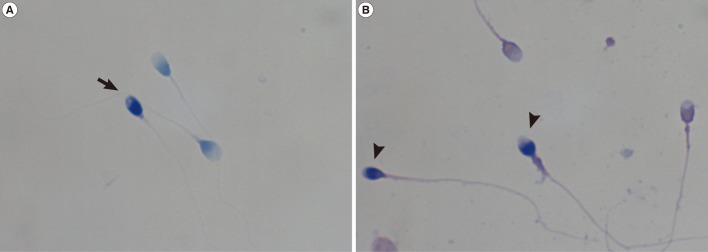
Figure 2
Correlation between the percentage of sperm cells with abnormal sperm chromatin condensation and structure.
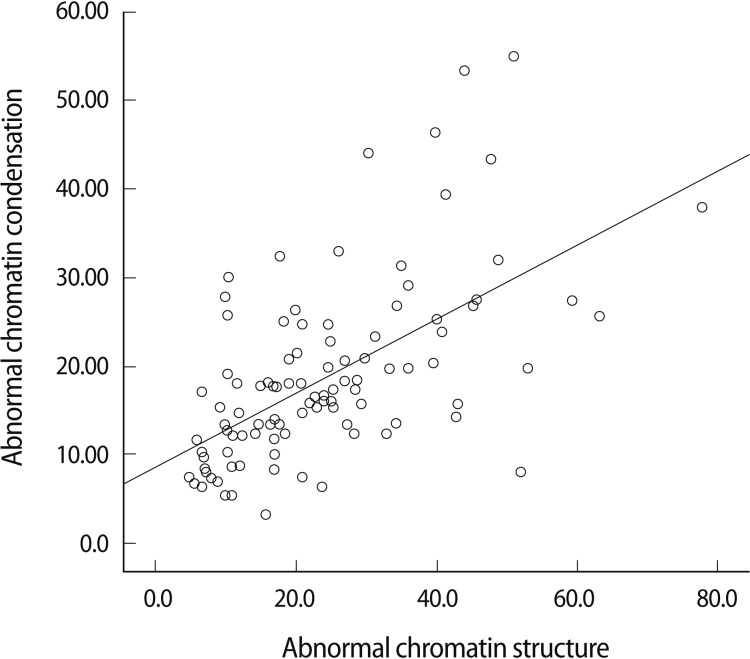
Figure 3
Correlation between the percentage of sperm cells with abnormal chromatin condensation and strict morphology.

Table 1
Mean percentage of sperm DNA integrity and chromatin condensation assessed by strict morphology