|
 |
- Search
| Clin Exp Reprod Med > Volume 39(4); 2012 > Article |
Abstract
Objective
Concerns are growing about the decrease in male reproductive health. Caffeine is one of the popular nutrients that has been implicated as a risk factor for infertility. In the present study, we examined whether in utero and lactational exposure to caffeine affects the reproductive function of the offspring of rats.
Methods
Pregnant rats received caffeine via drinking water during gestation (26 and 45 mg/kg) and lactation (25 and 35 mg/kg). Body and reproductive organ weight, seminiferous tubule diameter, germinal epithelium height, sperm parameters, fertility rate, number of implantations, and testosterone level of the offspring were assessed from birth to adulthood.
Results
Significant dose-related decreases were observed in the body and reproductive organ weight, seminiferous tubule diameter, and germinal epithelium height of the offspring. Sperm density had declined significantly in offspring of the low-dose and high-dose groups, by 8.81% and 19.97%, respectively, by postnatal day 150. The number of viable fetuses had decreased significantly in females mated with male offspring of the high-dose group at postnatal days 60, 90, 120, and 150. There were also significant reductions in testosterone levels of high-dose group offspring from birth to postnatal day 150.
In recent years, concerns have been growing about the decrease in male reproductive health as found in declining semen quantity and quality in developed countries [1-3]. It has been shown that exposure to environmental, occupational, lifestyle, and dietary factors can adversely affect reproduction. During recent decades, caffeine, one of the most comprehensively studied ingredients in the food supply, has been implicated in a number of epidemiologic studies as a risk factor for infertility [4], fetal loss [5], and fetal growth impairment [6,7]. Caffeine (1, 3, 7-trimethylxanthine) is a natural alkaloid found in the leaves, seeds, or fruits of at least 63 plant species worldwide and is part of a group of compounds known as methylxanthines. The most commonly known sources of caffeine are coffee, cocoa beans, kola nuts, and tea leaves [8]. Approximately 85% the world's population today uses substantial amounts of caffeine on a regular basis [9] and 80% of pregnant women consume caffeinated beverages [10].
Numerous studies have examined the effects of caffeine intake on male fertility. Caffeine has been reported to have deleterious effects on dogs' testicles [11] and produce degenerative changes in the testicles of rabbits that had been given coffee brew by a stomach tube for 3 to 4 weeks [12]. Atrophy of the testis and decreases in spermatogenesis have been described in 4- to 6-week-old male rats that had consumed methylxanthine (caffeine, theophylline, theobromine) for 14 to 75 weeks [13]. A commercial diet containing theobromine in amounts of 0.5, 1.0, and 1.5 percent have been shown to cause testicular alterations ranging from vacuolation of spermatids and spermatocytes to multinucleated cell formation and oligospermia or asospermia with extensive degeneration of seminiferous tubule cells [14]. It has been found that daily administration of caffeine (30 or 60 mg/kg) to mature male rabbits for four consecutive weeks caused an increase in plasma FSH and a decrease in LH [15]. In the same way, Parazzini et al. [16] reported an increasing risk of poor semen quality with increasing coffee consumption. However, Vine et al. [17] showed that caffeine intake does not appear to significantly affect sperm nuclear size, shape, or chromatin texture.
Furthermore, there are a number of research studies related to the developmental toxicity of caffeine in the reproductive system. Inhibition of Leydig cell differentiation and a significant decrease in their numbers were reported when caffeine (30 mg/kg per day) was administered to rats during pregnancy [18]. Ramlau-Hansen et al. [19] found that there was a tendency toward a decreasing semen volume with increasing maternal coffee consumption during pregnancy. It seems that exposure to caffeine during organogenesis may impact gonadal development and thus later gonadal function [19]. However, Eichler and Mugge [20] demonstrated that daily subcutaneous injections of caffeine did not result in a diminution of the reproductive capacity of albino rats over four generations.
Given the contradictory results on the reproductive effects of caffeine and the fact that caffeine is so widely consumed, its health benefits and consequences have been and are still being studied extensively. Furthermore, currently an in utero and lactational exposure method has been employed to identify the developmental toxicities of various chemicals [21,22]. Hence, the exposure period has been prolonged to examine the effects of lactational exposure in addition to the effects of gestational exposure. It is also important to examine the developmental toxicity of caffeine from birth until puberty. Therefore, since little is still known regarding the potential developmental toxicity of caffeine on the reproductive function of the male offspring, the present study was undertaken to investigate for the first time the long-term effects of in utero and lactational exposure to different doses of caffeine on reproductive parameters and fertility in male offspring rats.
The present study was supported with research grant 87/3/20/4093/42084 approved by the ethics committee of the Department of Biology, Faculty of Sciences, Shahid Chamran University of Ahvaz, Iran. Wistar rats were obtained from the animal house of Jondishapour Medical Sciences University of Ahvaz and kept under standard conditions on a constant 12-hour light/dark cycle and at a controlled temperature of 22±2℃. Standard pellet food and distilled water were available ad libitum.
Female Wistar rats (100±10 days old) were mated overnight at the proportion of three females per male. Vaginal smears were collected daily and examined for the presence of sperm. The day of sperm detection in the vaginal smears was considered to be day 0 of pregnancy. The pregnant rats were randomly divided into three equal groups: a control group and two treated groups, one that received a low dose and one that received a high dose of caffeine via drinking water during both gestation (26 and 45 mg/kg) and lactation (25 and 35 mg/kg). The doses were established from related reproductive and developmental studies [23,24]. These doses are within the range previously studied during development of rat offspring and shown to have behavioral effects on offspring when administered daily during gestation and lactation via drinking water to rats. Also, it has been reported that feeding ≥30 mg/kg/day of caffeine to rats harms male reproductive systems [18,25]. The doses were used in the present study are higher and lower than 30 mg/kg/day. Moreover, for a 70 kg human, 30 mg/kg would involve an intake of over 2 g caffeine (or more than 20 cups of coffee containing 100 mg caffeine/cup), a dose that is severely toxic and close to the lower limit of the estimated range of lethal doses [26]. However, if a correction is made for differences in the metabolic rate between rats and humans [27], a dose of 30 mg/kg for a rat converts to a much safer dose of 8.8 mg/kg (or about 6 or 7 cups of coffee) [28].
On postnatal days 1, 7, 21, 28, 60, 90, 120, and 150, five pups were randomly selected and weighed; under chloroform inhalation, anesthesia blood samples were collected and the rats' testes were removed, weighed, and fixed by immersion in Bouin's solution for 48 hours. On postnatal days 60, 90, 120, and 150, the fertility of individual males and quality of epididymal sperm were analyzed and the right epididymis, seminal vesicle, and ventral prostate were removed and weighed.
Tissue samples from the testes were excised and processed for paraffin embedding sections. Serial sections with a 5 µm thickness were stained with hematoxylin and eosin for morphometrical examination at a light microscopic scale. For measurement of seminiferous tubule diameter and germinal epithelium height, 90 round or nearly round cross-sections of seminiferous tubules of stages VII-VIII were randomly chosen in each rat. Then, using an ocular micrometer of light microscopy (Olympus BH, Tokyo, Japan), at a magnification of ×40, two perpendicular diameters of each cross-section of seminiferous tubules were measured and the mean of these was calculated. Also, the germinal epithelium height was measured in 4 equidistant parts of each seminiferous tubule cross-section and the mean of these was calculated.
The epididymis was separated carefully from the testis and divided into 3 segments: head, body, and tail. The epididymal tail was trimmed with scissors and placed in 1.0 mL of 0.1 M phosphate buffer of pH 7.4. It was then vigorously shaken for homogeneity and dispersal of sperm cells. The semen samples were assessed for motility, number, and gross morphology of sperms without the investigator knowing which samples were from which group. For analysis of sperm motility, an aliquot of 10 µL was placed in a hemocytometer chamber (Paul Marienfeld GmbH, Lauda-Konigshofen, Germany) and analyzed under a light microscope. One hundred sperm were evaluated per animal and classified as motile or immotile [29]. For evaluation of sperm density, a 10 µL aliquot of the epididymal sperm suspension was transferred to each counting chamber of the hemocytometer and allowed to stand for 5 minutes. The cells that settled during this time were counted by a light microscope at ×200 magnification. The sperm heads were counted and expressed as million/mL of suspension [29]. The sperm morphology was also determined using the eosin-nigrosin staining method. For this purpose 10 µL of 1% eosin Y and nigrosin was added to a test tube containing 40 µL of sperm suspension and mixed by gentle agitation. Then, the sperm were incubated at room temperature for 45 to 60 minutes for staining and then re-suspended with a Pasteur pipette [30]. Two hundred sperm per animal were examined microscopically at ×40 to ×100 magnifications and the number of morphologically abnormal sperm was recorded to give the percent abnormal sperm.
Blood samples were collected by heart puncture for determination of serum testosterone levels. The blood samples were left for 60 minutes to clot and then centrifuged for 10 minutes at ×2,430 g. The clear sera obtained were stored at -80℃ until the testosterone level was measured. The serum concentrations of testosterone were measured by using a diagnostic radioimmunoassay kit (Immunotech, Marseille, France).
On postnatal days 60, 90, 120, and 150, the male offspring from each group were paired individually with two adult females proven to be fertile and weighing 180 to 200 g. The successful mating was confirmed by the presence of a vaginal plug and spermatozoa in the vaginal smear. The mated females were laparotomized on day 15 post coitum and the numbers of implantation and/or resorption sites were recorded.
All data were analyzed using SPSS ver. 10.0 for Windows (SPSS Inc., Chicago, IL, USA). Reproductive parameters in different groups were compared by one-way analysis of variance. Differences were considered to be significant when p<0.05 and p<0.01, as specified in the results.
Treatment of dams with caffeine did not produce any visible signs of maternal toxicity during gestation or lactation. There were no statistically significant differences with regard to maternal body weight between the dams from the control group and the caffeine-treated groups during gestation and lactation. No significant difference was observed in the percent of pregnant dams that gave birth or the percent of live pups that survived to postnatal day 150. The general behavior of the male offspring was not affected by caffeine exposure during gestation or lactation.
The offspring of the high-dose group presented a lower body weight gain at all of the ages of postnatal development studied from birth to postnatal day 150. Significant reductions were seen in the body weights of the low-dose group of offspring on postnatal days 21, 28, 60, and 90 (Table 1).
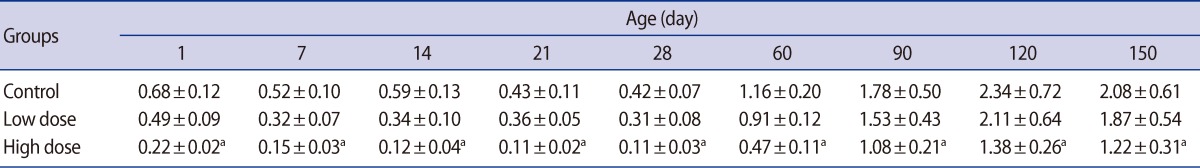
There were no significant differences in the absolute testis weight of the male offspring on postnatal days 1, 7, and 14 in the low-dose group or 1 and 7 in the high-dose group. The testis weight of the male offspring were significantly lower in the low-dose (p<0.05) and high-dose (p<0.01) groups compared with the control at the other ages of postnatal development that were studied (Table 2). Significant decreases were observed in the weight of the epididymis, ventral prostate, and seminal vesicle in the offspring of high-dose group on postnatal days 60, 90, 120, and 150. There were no statistically significant differences between low-dose and control groups' accessory gland weight of offspring throughout experiment (Table 3).
Histological analysis of the testes in the male offspring revealed that maternal caffeine consumption caused degenerative changes in the seminiferous tubule epithelium with loss of spermatogenesis in both treatment groups, particularly the high-dose one, at peripubertal (60 days old) and adult (90, 120, and 150 days old) phases of postnatal development (Figure 1).
Statistically significant reductions in the seminiferous tubule diameter were observed in the offspring of the low-dose and high-dose groups for the first time on postnatal days 14 and 7, respectively. These decreases in the seminiferous tubule diameter persisted in both of the caffeine-treated groups until postnatal day 150 (Table 4). Also, the germinal epithelium height decreased significantly in a dose-related manner in both treatment groups at all of the ages of postnatal development that were studied (Table 5).
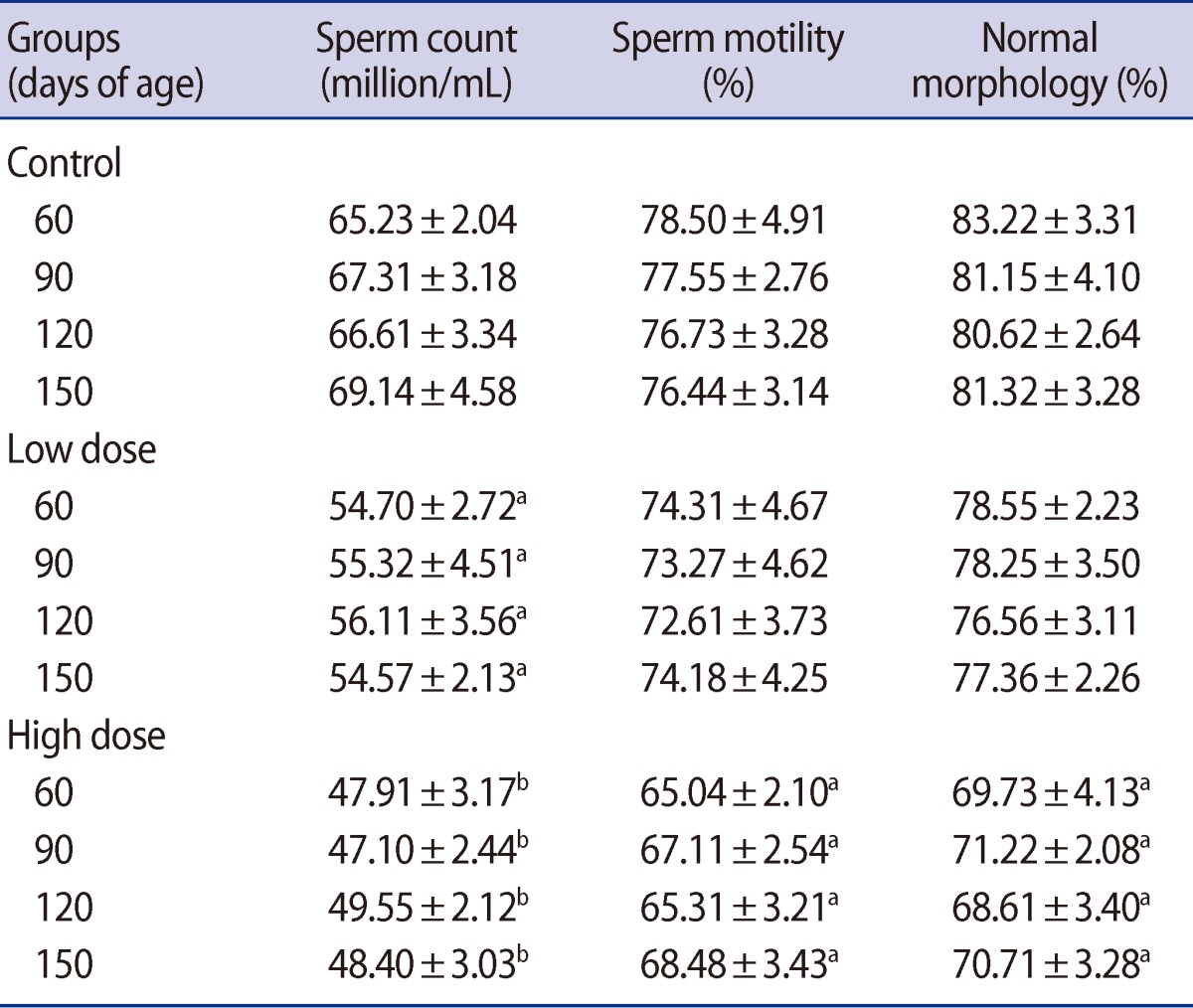
Sperm density was significantly lower in the offspring of both caffeine-treated groups at peripubertal (60 days old) and adult (90, 120, and 150 days old) phases of postnatal development (Table 6). A significant decrease was observed in the number of motile sperm in the offspring of the high-dose group compared with the control on postnatal days 60, 90, 120, and 150 (Table 6). Also, the percent of sperm with abnormal morphology was statistically higher in the offspring of the high-dose group on postnatal days 60, 90, 120, and 150 (Table 6). At none of the ages of postnatal development that were studied in the offspring was any significant difference observed between the low-dose and control groups' percentage of motile and morphologically normal sperm (Table 6).
Serum testosterone levels were significantly reduced in the offspring of the high-dose group on postnatal days 60, 90, 120, and 150. There were no statistically significant differences in the testosterone levels of the offspring between the low-dose and control groups at any studied ages of postnatal development (Table 7).
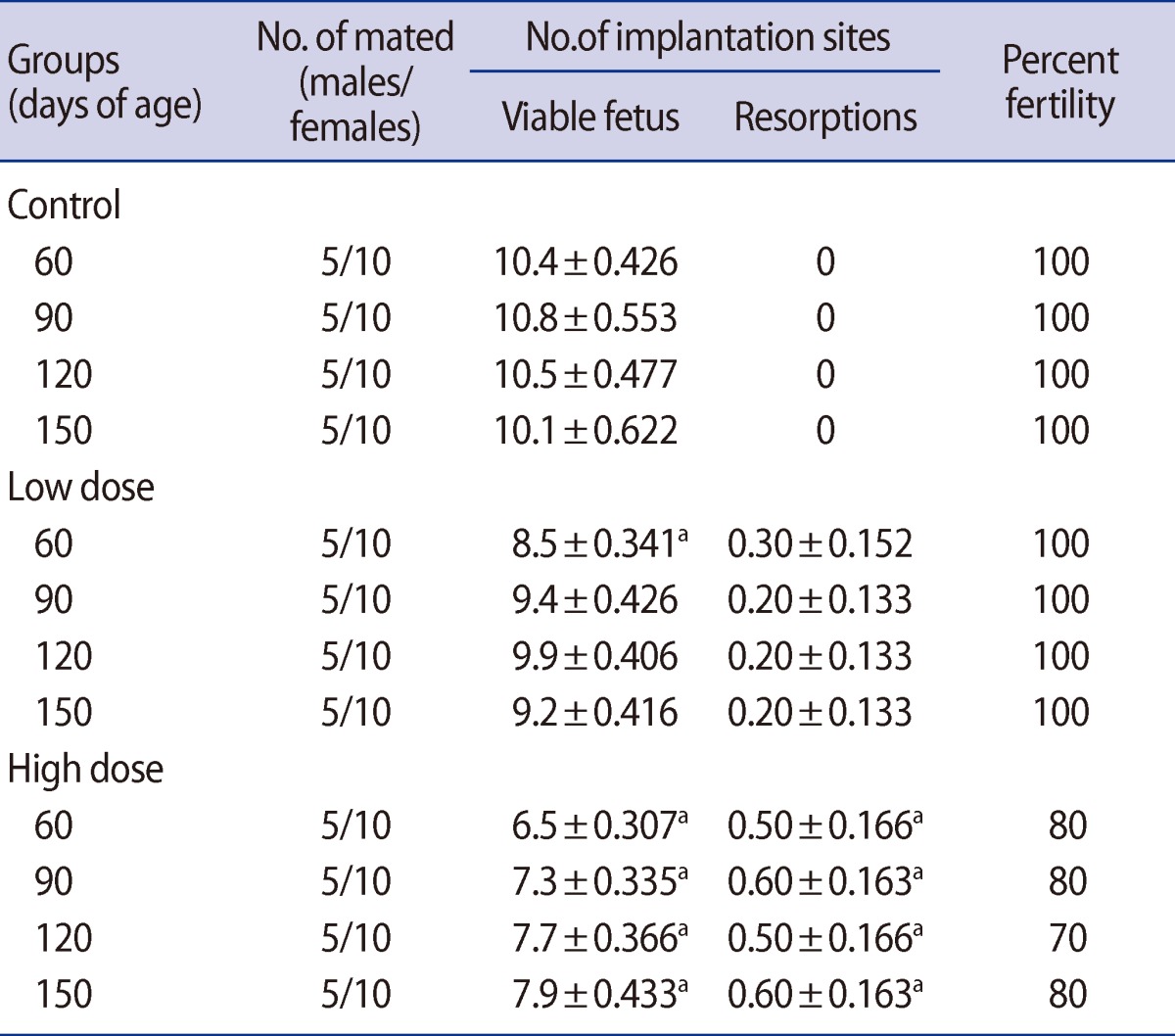
The fertility rate was reduced in the male offspring of the high-dose group compared with the control on postnatal days 60, 90, 120, and 150. Also in the male offspring, there were no observed differences between the low-dose and control groups' fertility rates (Table 8). Significant decreases were observed in the number of viable fetuses in females mated with the male offspring of the low-dose group on postnatal day 60 and in the high-dose group on postnatal days 60, 90, 120, and 150. The animals showed fetal resorption in both caffeine-treated groups throughout the experiment (Table 8).
In the present study, developmental toxicity of maternal caffeine consumption to the reproductive system was evaluated in growing male rat offspring. According to the results obtained from this study, maternal caffeine consumption can disrupt male reproductive development and impair fertility at later stages of life. Our findings demonstrate that gestational and lactational exposure to caffeine has adverse effects on body and reproductive organ weight, testicular structure, epididymal sperm parameters including sperm motility, sperm count and sperm morphology, serum testosterone levels, and fertility of male offspring rats. In addition, the present study indicates that the statistically significant reductions in reproductive parameters that have been observed in caffeine-treated offspring were not reversible after cessation of the exposure period and remained until postnatal day 150.
In the low-dose caffeine-treated group, the body weight of the offspring was significantly lower compared to the control group for a short period. However, the offspring of the high-dose treatment group had a significantly lower body weight from birth to postnatal day 150. This suggests that the reduction in the growth of male offspring depends on the amount of maternal caffeine intake. The decrease in the body weight of offspring observed in the present study is in consonance with earlier reports. Aeschbacher et al. [31] have examined three groups of Sprague-Dawley rats fed diets containing 0.25, 0.5, or 1 g caffeine/kg of diet throughout gestation and lactation. At the high level of caffeine, the birth weight of offspring was reduced and the pups exposed throughout gestation and lactation gained less weight during the preweaning period, but the two lower concentrations of caffeine had no effect on birth weight. Furthermore, Sprague-Dawley rats that were exposed to 5, 25, 50 or 75 mg/kg dosages of caffeine by gavage on days 3 to 19 of pregnancy showed a significant decrease in the body weight of their offspring at doses of 50 and 75 mg/kg [32]. Likewise, a daily administration of 15 or 35 mg/kg caffeine to monkeys (Macaca fascicularis) prior to and throughout pregnancy via drinking water caused a significant decrease in infant birth weight [33]. In addition, treatment of rats with 30 or 60 mg/kg of caffeine on days 2 to 20 of pregnancy resulted in reduced male pup weights with both the 30 and 60 mg/kg dosages through 19 weeks of age [34]. Vik et al. [35] suggested that a high caffeine intake in the third trimester of pregnancy caused fetal growth retardation, particularly in boys.
It is known that reproductive organ weights are crucial benchmarks for toxicological studies [36,37]. In our study, dose-related reductions were observed in the testis weight of offspring in both caffeine-treated groups. The testis weight on postnatal days 60 and 150 decreased by 11.09% and 5.61%, and 22.56% and 14.49% of the control values in the low-dose and high-dose groups, respectively. The weights of the epididymis, prostate, and seminal vesicle were also significantly affected by caffeine treatment in the offspring of the high-dose group. It is important to note that the weight of the testis is largely dependent on the mass of differentiated spermatogenic cells [38,39] and the weight of epididymis depends on the number of stored sperm in the epididymis. Caffeine seems to interfere with cell division resulting in a reduced number of spermatogenic cells [40,41]. As a result, the diameter of seminiferous tubules decreased significantly in offspring of both treatment groups during the prepubertal, postpubertal, and adulthood periods. The seminiferous tubule diameter decreased by 15.98% and 7.33% on day 60 and 29.53% and 15.39% on day 150 in the low-dose and high-dose groups, respectively. Pollard et al. [18] showed that caffeine, when administered during pregnancy, significantly inhibited differentiation of the seminiferous cords. However, it was found that 13-day-old fetal testis explants exposed to graded doses of caffeine or theobromine for 4 days in vitro differentiated normally, developing seminiferous cords made up of Sertoli and germ cells. In contrast, explants exposed to theophylline, another metabolite of caffeine, failed to develop seminiferous cords [42]. Moreover, atrophy of the seminiferous tubules with small patchy areas was reported in adult Albino rats that had been given 40 to 50 mg/kg/day caffeine [43]. Pollard and Smallshaw [44] reported that exposure of male Wistar rats to 30 mg/kg per day of caffeine, given by gavage for 15 to 38 consecutive days led to breakdown of the germinal epithelium. Daily administration of 30 or 60 mg/kg caffeine to mature male rabbits for four consecutive weeks caused a decrease in the sizes of the seminiferous tubules and inhibited spermatogenesis [15]. Severe bilateral testicular atrophy with aspermatogenesis or oligospermatogenesis was observed in 85 to 100 percent of 4- to 6-week-old male rats fed caffeine or theobromine for periods ranging from 14 to 75 weeks [13].
It has been suggested that the adequate bioavailability of testosterone plays an important role in the structural and functional integrity of the reproductive organs [45] and the decrease in weights of these organs could be due to inadequate circulating male hormone. A decreased testosterone level is also one of the indicators of chemical toxicity to the reproductive system [46]. In the present study, serum testosterone in the offspring of the high-dose treatment group markedly decreased during the prepubertal, postpubertal, and adulthood periods. Pollard et al. [18] reported that exposure to 30 mg/kg per day of caffeine during pregnancy significantly inhibited differentiation of the interstitial tissue and Leydig cells, decreased the number of Leydig cells exhibiting 3 beta-hydroxysteroid dehydrogenase activity, and also reduced biosynthesis of testosterone in the fetal testes at days 15 and 16 of gestation. However, it was found that 13-day-old fetal testis explants exposed to graded doses of caffeine or theobromine for 4 days in vitro differentiated functionally active Leydig cells appearing in the newly formed interstitium. In contrast, explants exposed to theophylline, another metabolite of caffeine, failed to develop normal Leydig cells [42]. Furthermore, it has been shown that the sons of mothers drinking 4-7 cups of coffee per day had lower testosterone levels than the sons of mothers drinking 0-3 cups per day (p<0.04) [19]. Ezzat and el-Gohary [15] observed an increase in plasma FSH, a decrease in LH, and an increase in testosterone levels after administration of caffeine to mature rabbits.
In addition, it is known that testosterone is essential for maintaining the structure and function of the male accessory sex glands. Accessory glands such as the prostate and seminal vesicles require androgen for differentiation, development, and maintenance of epithelial cells [47]. Therefore, it can be interpreted that the decrease in the weights of prostate and seminal vesicles of male offspring may be related to reduced levels of testosterone.
Also, the results showed that maternal caffeine consumption impairs sperm quality in male offspring. One of the most sensitive tests for evaluating spermatogenesis is sperm count in the epididymis [48]. Caffeine treatment caused a decrease in sperm density and percent of motile and morphologically normal sperm in offspring during the postpubertal and adulthood periods. Sperm production decreased 14.83% and 8.81% on days 60 and 25.40% and 19.97% on days 150 in low-dose and high-dose groups, respectively. The reduction in epididymal sperm count may be due to an adverse effect of caffeine on spermatogenesis. It has been reported that sperm counts may decrease as a result of a reduction in testosterone levels [25]. These changes in epididymal sperm counts are consistent with changes in epididymal weights. Additionally, it appears that caffeine caused genetic damage to the spermatozoa where nucleic cysts and pouches were observed at ultrastructural levels [44].
A significant decrease in sperm counts of male offspring rats were observed, leading to significant decreases in the number of viable fetuses in females mated with the male offspring. Meistrich et al. [49] have also reported that a sample containing a high percentage of abnormal sperm is indicative of impaired fertility.
Ax et al. [40] showed that in the testes of roosters fed a diet containing 145 mg/kg caffeine for 35 days, the semen output and sperm concentration were markedly reduced 17 to 21 days after treatment, and no semen could be collected from the roosters after they had received caffeine for 30 days. Moreover, Margalioth et al. [50] demonstrated that while exposure to caffeine enhanced sperm motility, the ability of the sperm to penetrate hamster oocytes was not improved. A significant reduction in the abundance of mature spermatozoa was also seen in Wistar rats that received 30 mg/kg per day of caffeine, given by gavage for 15 to 38 consecutive days [44]. Marshburn et al. [51] found that sperm motility decreased in men who consumed more than 2 cups of coffee per day. Washing and incubation of human spermatozoa with 6 µm caffeine caused a 45% decline in the percentage of progressively motile spermatozoa and a very small decrease in their velocity [52]. Vine et al. [17] reported that caffeine intake and alcohol consumption do not appear to significantly affect sperm nuclear size, shape, or chromatin texture. Tsuzuki and Fujihara [53] indicated that bovine sperm motility decreased after 6 hours incubation with caffeine. Ramlau-Hansen et al. [19] showed there was a tendency toward decreasing crude median semen volume (p<0.06) with increasing maternal coffee consumption during pregnancy. The crude median sperm concentration and total sperm count among sons of mothers consuming 7 cups of coffee per day were 33 million/mL and 101 million, which are, respectively, 21% and 28% lower than the crude medians among sons of mothers consuming 0 to 3 cups/day.
The precise mechanism underlying caffeine-induced male reproductive toxicity remains unknown. The possible mechanism for reproductive toxicity of caffeine is an agonistic or antagonistic effect on the adenosine, adrenergic, cholinergic gamma-amino butyric acid, or serotonin receptors [54]. However, further research is needed to clarify the developmental effects of caffeine and its metabolites during an extended period, using a larger number of animals and in female offspring. Consequently, the present study indicates that prenatal and early neonatal life comprises a sensitive period for induction of permanent adverse effects by caffeine on reproductive parameters of male offspring rats. Therefore, maternal caffeine consumption affects development of the reproductive system and has deleterious long-term effects on reproductive efficiency and fertility of male offspring in the peripubertal, postpubertal, and adulthood periods.
Notes
References
1. Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. Evidence for decreasing quality of semen during past 50 years. BMJ 1992;305:609-613.PMID: 1393072.



2. Comhaire F, Waeleghem KV, De Clercq N, Schoonjans F. Declining sperm quality in European men. Andrologia 1996;28:300-301.PMID: 9021037.


3. Olesen IA, Sonne SB, Hoei-Hansen CE, Rajpert-DeMeyts E, Skakkebaek NE. Environment, testicular dysgenesis and carcinoma in situ testis. Best Pract Res Clin Endocrinol Metab 2007;21:462-478.PMID: 17875492.


4. Wilcox A, Weinberg C, Baird D. Caffeinated beverages and decreased fertility. Lancet 1988;2:1453-1456.PMID: 2904572.


5. Srisuphan W, Bracken MB. Caffeine consumption during pregnancy and association with late spontaneous abortion. Am J Obstet Gynecol 1986;154:14-20.PMID: 3946486.


6. Martin TR, Bracken MB. The association between low birth weight and caffeine consumption during pregnancy. Am J Epidemiol 1987;126:813-821.PMID: 3661529.


7. Fenster L, Eskenazi B, Windham GC, Swan SH. Caffeine consumption during pregnancy and fetal growth. Am J Public Health 1991;81:458-461.PMID: 2003624.



9. Harris M. The Buzz on caffeine. Veg Times 2004;317:71-73.
10. Stanton CK, Gray RH. Effects of caffeine consumption on delayed conception. Am J Epidemiol 1995;142:1322-1329.PMID: 7503053.


11. Vacca G. Riserche sulla alterazioni testicolari nell' awelanameuto speri mentale da caffeina. Arch Farmacol Sper Sci Affin 1926;42:62.
12. Stieve H. Untersuchungen ueber die wechselbeziehungen zwischen gesamt korper und keimdrusen. Akad Verl Ges 1931;23:571-594.
13. Friedman L, Weinberger MA, Farber TM, Moreland FM, Peters EL, Gilmore CE, et al. Testicular atrophy and impaired spermatogenesis in rats fed high levels of the methylxanthines caffeine, theobromine, or theophylline. J Environ Pathol Toxicol 1979;2:687-706.PMID: 422930.

14. Soffietti MG, Nebbia C, Valenza F, Amedeo S, Re G. Toxic effects of theobromine on mature and immature male rabbits. J Comp Pathol 1989;100:47-58.PMID: 2918109.


15. Ezzat AR, el-Gohary ZM. Hormonal and histological effects of chronic caffeine administration on the pituitary-gonadal and pituitary-adrenocortical axes in male rabbits. Funct Dev Morphol 1994;4:45-50.PMID: 7819609.

16. Parazzini F, Marchini M, Tozzi L, Mezzopane R, Fedele L. Risk factors for unexplained dyspermia in infertile men: a case-control study. Arch Androl 1993;31:105-113.PMID: 8215689.


17. Vine MF, Setzer RW Jr, Everson RB, Wyrobek AJ. Human sperm morphometry and smoking, caffeine, and alcohol consumption. Reprod Toxicol 1997;11:179-184.PMID: 9100290.


18. Pollard I, Williamson S, Magre S. Influence of caffeine administered during pregnancy on the early differentiation of fetal rat ovaries and testes. J Dev Physiol 1990;13:59-65.PMID: 2283461.

19. Ramlau-Hansen CH, Thulstrup AM, Bonde JP, Olsen J, Bech BH. Semen quality according to prenatal coffee and present caffeine exposure: two decades of follow-up of a pregnancy cohort. Hum Reprod 2008;23:2799-2805.PMID: 18757446.


20. Eichler O, Mugge H. Zur frage der schadlichkeit des coffeins bei chronischer zufuhr. Arch Exp Path Pharmakol 1932;168:89.

21. Moore RW, Rudy TA, Lin TM, Ko K, Peterson RE. Abnormalities of sexual development in male rats with in utero and lactational exposure to the antiandrogenic plasticizer Di(2-ethylhexyl) phthalate. Environ Health Perspect 2001;109:229-237.PMID: 11333183.



22. Odum J, Lefevre PA, Tinwell H, Van Miller JP, Joiner RL, Chapin RE, et al. Comparison of the developmental and reproductive toxicity of diethylstilbestrol administered to rats in utero, lactationally, preweaning, or postweaning. Toxicol Sci 2002;68:147-163.PMID: 12075118.


23. Hughes RN, Beveridge IJ. Behavioral effects of exposure to caffeine during gestation, lactation or both. Neurotoxicol Teratol 1991;13:641-647.PMID: 1779952.


24. Hughes RN, Loader VG. Effects on elevated plus-maze behavior of exposure to caffeine during both gestation and lactation. Psychobiology 1996;24:314-319.


25. Uzunhisarcikli M, Kalender Y, Dirican K, Kalender S, Ogutcu A, Buyukkomurcu F. Acute, subacute and subchronic administration of methyl parathioninduced testicular damage in male rats and protective role of vitamins C and E. Pestic Biochem Physiol 2007;87:115-122.

26. McKim WA. Drugs and behavior: an introduction to behavioral pharmacology. 2007. 6th ed. Upper Saddle River, NJ: Pearson Education Inc.
27. Nehlig A, Debry G. Potential teratogenic and neurodevelopmental consequences of coffee and caffeine exposure: a review on human and animal data. Neurotoxicol Teratol 1994;16:531-543.PMID: 7862054.


28. Anderson NL, Hughes RN. Increased emotional reactivity in rats following exposure to caffeine during adolescence. Neurotoxicol Teratol 2008;30:195-201.PMID: 18378115.


29. Seed J, Chapin RE, Clegg ED, Dostal LA, Foote RH, Hurtt ME, et al. ILSI Risk Science Institute Expert Working Group on Sperm Evaluation. Methods for assessing sperm motility, morphology, and counts in the rat, rabbit, and dog: a consensus report. Reprod Toxicol 1996;10:237-244.PMID: 8738562.


30. Narayana K, Prashanthi N, Nayanatara A, Kumar HH, Abhilash K, Bairy KL. Effects of methyl parathion (o,o-dimethyl o-4-nitrophenyl phosphorothioate) on rat sperm morphology and sperm count, but not fertility, are associated with decreased ascorbic acid level in the testis. Mutat Res 2005;588:28-34.PMID: 16226487.


31. Aeschbacher HU, Milon H, Poot A, Würzner HP. Effect of caffeine on rat offspring from treated dams. Toxicol Lett 1980;7:71-77.PMID: 7292516.


32. West GL, Sobotka TJ, Brodie RE, Beier JM, O'Donnell MW Jr. Postnatal neurobehavioral development in rats exposed in utero to caffeine. Neurobehav Toxicol Teratol 1986;8:29-43.PMID: 3703093.

33. Gilbert SG, So Y, Klassen RD, Geoffroy S, Stavric B, Rice DC. Elimination of chronically consumed caffeine in the pregnant monkey (Macaca fascicularis). J Pharmacol Exp Ther 1986;239:891-897.PMID: 3795047.

34. Tye K, Pollard I, Karlsson L, Scheibner V, Tye G. Caffeine exposure in utero increases the incidence of apnea in adult rats. Reprod Toxicol 1993;7:449-452.PMID: 8274820.


35. Vik T, Bakketeig LS, Trygg KU, Lund-Larsen K, Jacobsen G. High caffeine consumption in the third trimester of pregnancy: gender-specific effects on fetal growth. Paediatr Perinat Epidemiol 2003;17:324-331.PMID: 14629313.


36. Crissman JW, Goodman DG, Hildebrandt PK, Maronpot RR, Prater DA, Riley JH, et al. Best practices guideline: toxicologic histopathology. Toxicol Pathol 2004;32:126-131.PMID: 14713558.


37. Yavasoglu A, Karaaslan MA, Uyanikgil Y, Sayim F, Ates U, Yavasoglu NU. Toxic effects of anatoxin-a on testes and sperm counts of male mice. Exp Toxicol Pathol 2008;60:391-396.PMID: 18514498.


38. Boockfor FR, Blake CA. Chronic administration of 4-tert-octylphenol to adult male rats causes shrinkage of the testes and male accessory sex organs, disrupts spermatogenesis, and increases the incidence of sperm deformities. Biol Reprod 1997;57:267-277.PMID: 9241039.


39. Mruk DD, Cheng CY. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev 2004;25:747-806.PMID: 15466940.


40. Ax RL, Collier RJ, Lodge JR. Effects of dietary caffeine on the testis of the domestic fowl, Gallus domesticus. J Reprod Fertil 1976;47:235-238.PMID: 957321.


41. Kaukab N, Saeed M. Effects of caffeine on rat testes. Prof Med J 1999;6:1-5.
42. Pollard I, Locquet O, Solvar A, Magre S. Effects of caffeine and its reactive metabolites theophylline and theobromine on the differentiating testis. Reprod Fertil Dev 2001;13:435-441.PMID: 11833941.


43. Bachmann G, Haldi J, Wynn W, Ensor C. Reproductivity and growth of albino rats on a prolonged daily intake of caffeine. J Nutr 1946;32:239-247.PMID: 20999571.


44. Pollard I, Smallshaw J. Male mediated caffeine effects over two generations of rats. J Dev Physiol 1988;10:271-281.PMID: 3216096.

45. Mann T. Secretory function of the prostate, seminal vesicle and other male accessory organs of reproduction. J Reprod Fertil 1974;37:179-188.PMID: 4593605.


46. Yoshida M, Kitani T, Takenaka A, Kudoh K, Katsuda SI, Taya K, et al. Lack of effects of oxolinic acid on spermatogenesis in young adult and aged Wistar rats. Food Chem Toxicol 2002;40:1815-1825.PMID: 12419696.


47. Ono Y, Suzuki K, Kashiwagi B, Shibata Y, Ito K, Fukabori Y, et al. Role of androgen on blood flow and capillary structure in rat seminal vesicles. Tohoku J Exp Med 2004;202:193-201.PMID: 15065645.


48. Chandra AK, Ghosh R, Chatterjee A, Sarkar M. Effects of vanadate on male rat reproductive tract histology, oxidative stress markers and androgenic enzyme activities. J Inorg Biochem 2007;101:944-956.PMID: 17475337.


49. Meistrich ML, Goldstein LS, Wyrobek AJ. Long-term infertility and dominant lethal mutations in male mice treated with adriamycin. Mutat Res 1985;152:53-65.PMID: 4047085.


50. Margalioth EJ, May JY, Navot D, Laufer N, Ovadia J, Schenker JG. Effect of caffeine on human sperm penetration into zona-free hamster ova. Arch Androl 1985;14:139-142.PMID: 3840673.


51. Marshburn PB, Sloan CS, Hammond MG. Semen quality and association with coffee drinking, cigarette smoking, and ethanol consumption. Fertil Steril 1989;52:162-165.PMID: 2744185.


52. Rees JM, Ford WC, Hull MG. Effect of caffeine and of pentoxifylline on the motility and metabolism of human spermatozoa. J Reprod Fertil 1990;90:147-156.PMID: 2231536.


53. Tsuzuki Y, Fujihara N. The effects of caffeine on sperm motility and in vitro embryo development for different bulls. J Mamm Ova Res 1998;15:27-30.

54. Brent RL, Christian MS, Diener RM. Evaluation of the reproductive and developmental risks of caffeine. Birth Defects Res B Dev Reprod Toxicol 2011;92:152-187.PMID: 21370398.



Figure 1
Histological sections of the testis (H&E, scale bar: 200 µm) and morphology of sperm (eosin-nigrosin, ×40) in male offspring rats at 90 days of age in control (A, B) and caffeine-treated groups (C, D, E). Atrophy and degeneration of seminiferous tubules and loss of spermatogenesis, decrease in spermatogenic cell layer and germinal epithelium height (arrows), increase of tubular lumen and presence of vacuoles in germinal epithelium, and also sperm with abnormal morphology; bent midpiece and tail (arrows) were seen in both treatments, particularly in the high-dose, groups. GE, germinal epithelium.
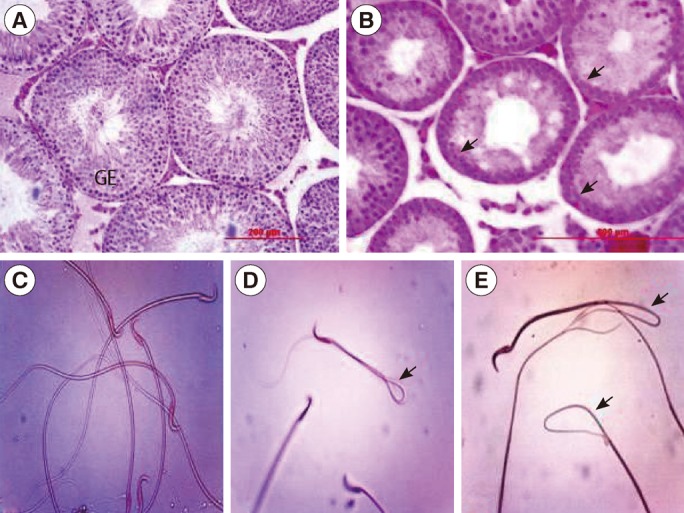
Table 1
Mean (±SE) of body weight (g) in offspring rats of control and caffeine-treated groups at different ages of postnatal development
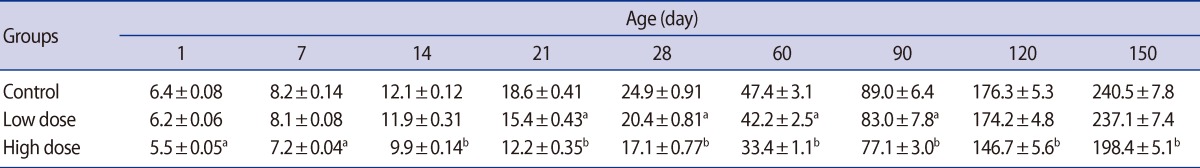
Table 2
Mean (±SE) of testis weight (mg) in offspring rats of control and caffeine-treated groups at different ages of postnatal development
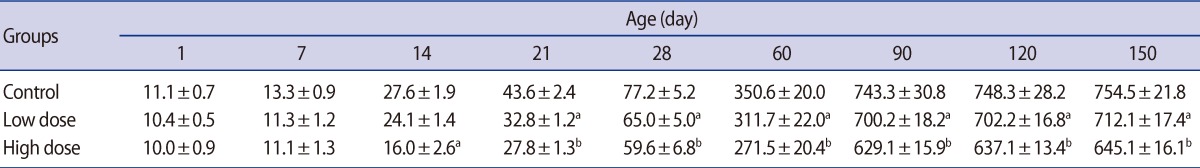
Table 3
Mean (±SE) of epididymis, ventral prostate and seminal vesicle weight (mg) in offspring rats of control and caffeine-treated groups at different ages of postnatal development
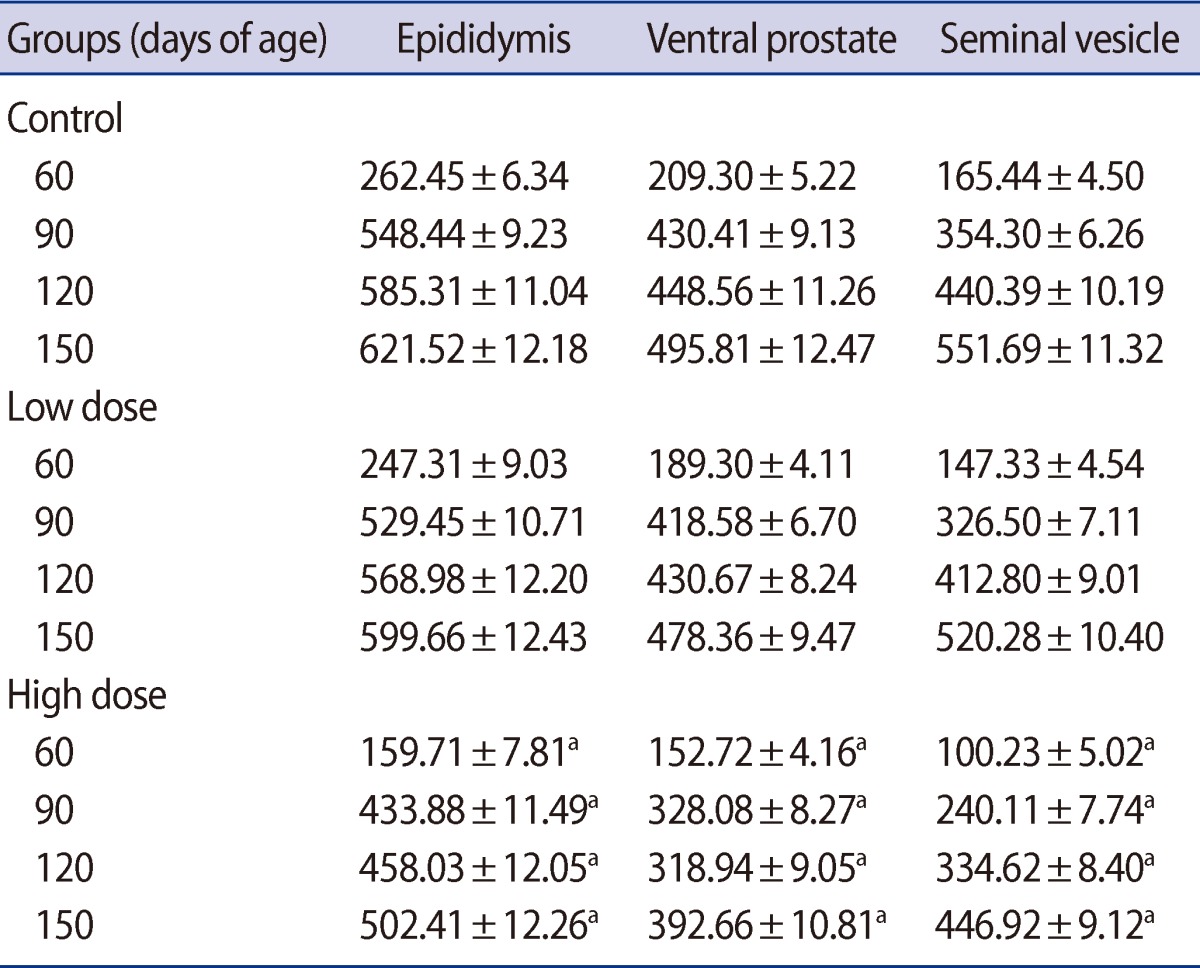
Table 4
Mean (±SE) of seminiferous tubules diameter (µm) in offspring rats of control and caffeine-treated groups at different ages of postnatal development
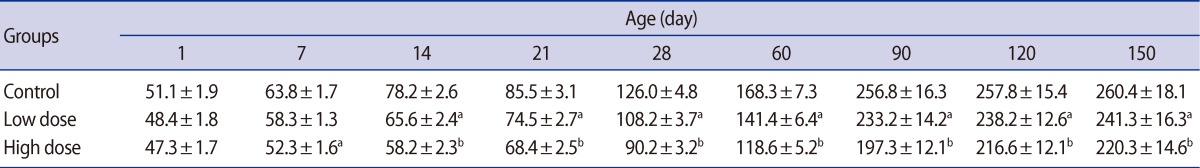
Table 5
Mean (±SE) of germinal epithelium height (µm) in offspring rats of control and caffeine-treated groups at different ages of postnatal development
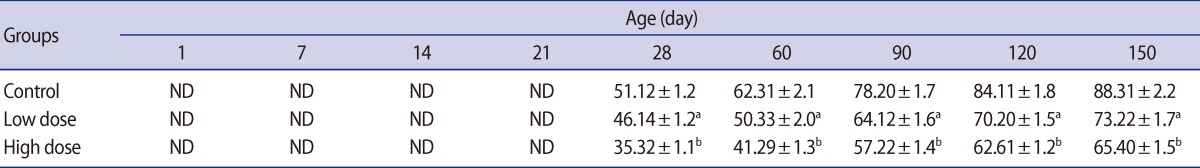
Table 6
Mean (±SE) of sperm motility (%), sperm density (million/mL) and percent of sperm with normal morphology (%) in offspring rats of control and caffeine-treated groups at different ages of postnatal development