Variable localization of Toll-like receptors in human fallopian tube epithelial cells
Article information
Abstract
Objective
To determine the localization, expression, and function of Toll-like receptors (TLRs) in fallopian tube epithelial cells.
Methods
The localization of TLRs in fallopian tube epithelial cells was investigated by immunostaining. Surprisingly, the intensity of staining was not equal in the secretory and ciliated cells. After primary cell culture of fallopian tube epithelial cells, ring cloning was used to isolate colonies of ciliated epithelial cells, distinct from non-ciliated epithelial cells. The expression of TLRs 1–10 was examined by quantitative real-time polymerase chain reaction, and protein localization was confirmed by immunostaining. The function of the TLRs was determined by interleukin (IL)-6 and IL-8 production in response to TLR2, TLR3, TLR5, TLR7, and TLR9 ligands.
Results
Fallopian tube epithelial cells expressed TLRs 1–10 in a cell-type-specific manner. Exposing fallopian tube epithelial cells to TLR2, TLR3, TLR5, TLR7, and TLR9 agonists induced the secretion of proinflammatory cytokines such as IL-6 and IL-8.
Conclusion
Our findings suggest that TLR expression in the fallopian tubes is cell-type-specific. According to our results, ciliated cells may play more effective role than non-ciliated cells in the innate immune defense of the fallopian tubes, and in interactions with gametes and embryos.
Introduction
The fallopian tube, as a dynamic reproductive organ, plays an important role in gamete transport, the final maturation of female and male gametes, fertilization, early development of the embryo, and transport of the embryo to the uterus [1]. From the histological point of view, the fallopian tube consists of three layers: the mucosa, muscular, and serosa layers. The tubal mucosa is composed of cuboidal or simple columnar epithelium made up of ciliated and secretory cells. Ciliated cells are seen predominantly on the apex of the mucosal folds [2]. They undergo cyclical changes in morphology and ciliary activity under the influence of estrogen and progesterone [13].
Sexually transmitted infections (STIs) are a major clinical concern in reproductive medicine because of their reproductive sequelae [456]. For example, genital Chlamydia trachomatis infection, particularly within the fallopian tubes, can have serious consequences, such as pelvic inflammatory disease, tubal factor infertility, and pregnancy complications [578]. The effects of the immune system related to epithelial cells of the female reproductive tract represent the first reaction to sexually transmitted bacterial and viral pathogens [9].
During the last decade, Toll-like receptors (TLRs) have been identified as principal sentinels of the innate immune system that possess the ability to recognize both pathogen-associated molecular patterns (PAMPs) and endogenous damage-associated molecular patterns. The TLR family also links innate and adaptive immunity through the production of proinflammatory cytokines and further specialist immune cell recruitment [10111213]. Recently, an analysis of 10 human and 12 mice TLRs found that they can be grouped into two categories based on their localization or their activation by microbial membrane lipids, microbial nucleic acids, or bacterial proteins [1214].
TLR1, TLR2, TLR4, TLR5, TLR6, and TLR10 are located on the plasma membrane, whereas TLR3, TLR7, TLR8, and TLR9 are expressed in cytoplasmic organelles, mainly the endosomes, lysosomes, endolysosomes, and endoplasmic reticulum [1516]. The TLRs located on the plasma membrane respond to bacterial PAMPs. In contrast, TLR3 detects viral double-stranded RNA, TLR7 and TLR8 react to self and viral single-strand RNA, and TLR9 binds unmethylated bacterial DNA. It remains unknown which ligands activate TLR10 [9].
TLRs trigger signal transduction through adaptor molecules that are recruited to the intracellular domain of the TLR upon ligand binding. Among them, MyD88 is widely utilized by all TLRs, with the exception of TLR3. TRIF is an adaptor molecule for TLR3 and TLR4, while the MyD88-adapter-like adaptor mediates signaling from TLR2 and TLR4. In contrast, the TRIF-related adaptor molecule is exclusively recruited by TLR4. Finally, these signaling pathways activate the transcription factor NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) and activator protein-1, which is common to all TLRs, leading to the production of inflammatory mediators such as interleukin (IL)-6 and IL-8. TLR3, TLR4, TLR7, TLR8, and TLR9 also activate interferon regulatory factor (IRF) 3 and/or IRF7, leading to the production of interferon (IFN)-β and IFN-α [17].
The presence of TLRs in the upper female reproductive tract has been established in several studies [918]. TLR expression is menstruation-dependent, and TLRs are involved in the regulation of ovulation, sperm capacitation, fertilization, gestation, and parturition, as well as in pathological and inflammatory conditions such as endometritis and STIs [919202122].
To our knowledge, the present study is the first to investigate whether there are immunological differences between the two cell types of the fallopian tube. In light of the pivotal role of TLRs in innate immunity and the reproductive significance of the fallopian tubes, we explored the expression and function of TLRs in ciliated and non-ciliated human fallopian tube epithelial cells.
Methods
1. Patients and samples
The current study was approved by the Ethics Committee of ROYAN Institute and written informed consent was obtained prior to the collection of tissue samples. Fallopian tube tissue samples were collected from nine patients undergoing tubal ligation or total abdominal hysterectomy for benign gynecological conditions.
Fallopian tube tissues were transported from the operating theater in Hanks' balanced salt solution (Gibco Life Technologies, Paisley, UK). A small section of the fallopian tubes (0.5 cm) was cut and divided into two pieces. The first portion was fixed in formalin for immunohistochemistry. The other part of each sample was used for the primary cell culture.
2. Antibodies and peptides
Antibodies and peptides were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). These were goat polyclonal antibodies specific for the N-terminal domains of TLR1, TLR2, TLR3, TLR5, TLR6, TLR7, and TLR9 (sc8687, sc8689, sc8691, sc8695, sc5657, sc13207, and sc13212, respectively), goat polyclonal antibody specific for the C-terminal domains of TLR4 (sc8694), goat polyclonal antibody specific for the V-terminal domains of TLR10 (sc23577), and rabbit polyclonal antibody specific for the D-terminal domains of TLR8 (sc-13212-R). Blocking peptides specific for the respective antibodies were used to detect non-specific staining.
3. Immunostaining
The tissue samples were fixed in 10% formalin, embedded in paraffin, cut into 4-µm sections, and stained with hematoxylin and eosin following a routine protocol. The sections were deparaffinized with xylosine, followed by rehydration in graded ethanol. Endogenous peroxidase activity was blocked with 3% by volume hydrogen peroxidase in methanol. Antigen retrieval was performed by microwave irradiation in 10 mmol/L sodium citrate (pH 6.0), followed by washing in phosphate-buffered saline (PBS) and staining using a Vectastain Elite ABC peroxidase kit (Vector Laboratories, Peterborough, UK). In addition, to avoid non-specific binding, an avidin/biotin blocking kit was used. The block was then removed and the slides were incubated overnight with a primary antibody. Binding was visualized by incubation with the peroxidase substrate 3-amino-9-ethylcarbazole and counterstained with 10% hematoxylin. Negative controls were obtained by blocking the primary antibody with the corresponding specific peptide. Immunostained sections were examined using an Olympus BH2 microscope (Tokyo, Japan) [23].
4. Primary cell culture of epithelial cells
For primary cell culture, the fallopian tube tissues were cut longitudinally and layered, then incubated with 0.25% collagenase (Sigma-Aldrich, Poole, UK). After 1 hour, the epithelial cells were scraped off each tube gently and washed with DMEM/F12 culture medium (Invitrogen, Paisley, UK) supplemented with 1% penicillin and streptomycin (Sigma-Aldrich), 10% fetal calf serum (FCS, Invitrogen) and 1% Lglutamine (Invitrogen). After centrifugation, the pellet of cells was resuspended in normal medium and cultured in a 6-well plate culture dish until confluency. Confluent cells were subcultured on a chamber slide for cytokeratin immunocytochemical staining and in T75 culture flasks until confluency [23].
5. Cytokeratin immunostaining
For immunostaining, epithelial cells were cultured in 4-well chamber slides at 37℃ in DMEM/F12 culture medium (Invitrogen) supplemented with 1% penicillin and streptomycin (Sigma-Aldrich), 10% FCS (Invitrogen) and L-glutamine (Invitrogen) in a 5% CO2 atmosphere. At confluency, the slides were washed with PBS, fixed with 5% formalin, and stored at 4℃. Staining for cytokeratin was performed using a Vectastain Elite ABC peroxidase kit (Vector Laboratories). To avoid non-specific binding, an avidin/biotin blocking kit (Vector Laboratories) was used. After removing the block, the slides were incubated for 2 hours at room temperature in primary antibody (monoclonal anti-pan cytokeratin antibody clone C-11, Sigma-Aldrich) at an appropriate dilution using antibody diluent medium (Dakocytomation Ltd., Cambridgeshire, UK) and 250 mL of biotin per milliliter of diluted antibody. Binding was visualized by incubation with DAB substrate (Vector Laboratories), and the slides were then counterstained with hematoxylin and mounted with DPX (VWR International, Lutterworth, UK).
6. Isolation of ciliated from non-ciliated epithelial cells
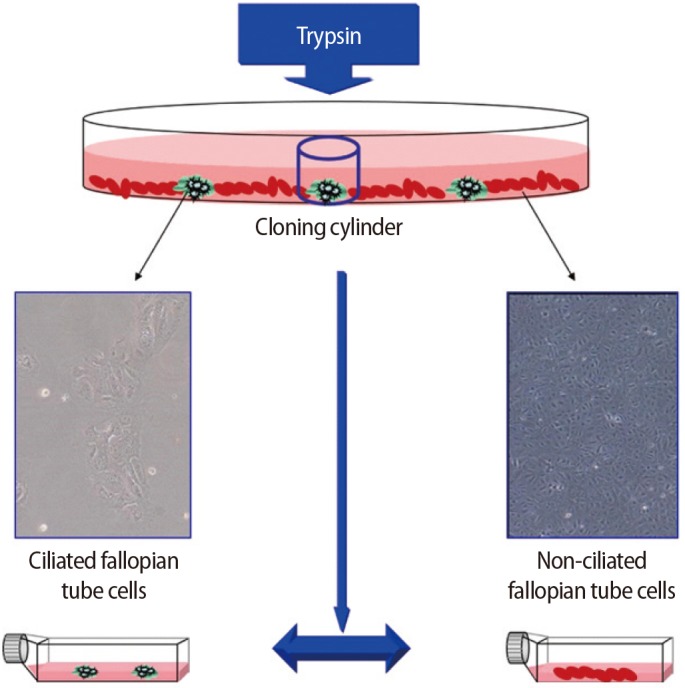
Ring cloning was used to isolate colonies of ciliated epithelial cells from non-ciliated epithelial cells. Briefly, a cloning cylinder with one edge covered with sterile silicon wax was placed on a colony of ciliated epithelial cells, and 10 µL of trypsin was used to remove the ciliated epithelial cells. The cells were then transferred to a 12-well plate and grown to confluency (Figure 1).
Ciliated and non-ciliated cells of the fallopian tubes were separately subcultured at 37℃ in DMEM/F12 culture medium (Invitrogen) supplemented with 1% penicillin and streptomycin (Sigma-Aldrich), 10% FCS (Invitrogen), and 1% L-glutamine (Invitrogen) in a 5% CO2 atmosphere until confluency (5 days).
7. RNA isolation and cDNA synthesis
For genomic studies, ciliated and non-ciliated epithelial cells were washed with Dulbecco's PBS without Ca2+ or Mg2+, harvested using trypsin-EDTA (Invitrogen), and pelleted by centrifugation at 300×g for 5 minutes, at which point the supernatant was discarded. One milliliter of TRI reagent (Sigma-Aldrich) was added onto the pellet (5×106 cells). Thereafter, the total RNA from the pelleted cells was extracted following the standard protocol supplied by the manufacturer.
The total RNA obtained from ciliated and non-ciliated epithelial cells was treated three times with DNase I (Fermentas, Sankt Leon-Rot, Germany) to remove genomic DNA contamination from the samples. First-strand cDNA synthesis was performed using oligo dT primers (Fermentas kit) and reverse transcription by Super-Script II (200 Ul, Invitrogen). Negative controls were prepared without inclusion of the enzyme (non-reverse transcribed [non-RT] controls, RT controls).
8. Quantitative real-time polymerase chain reaction
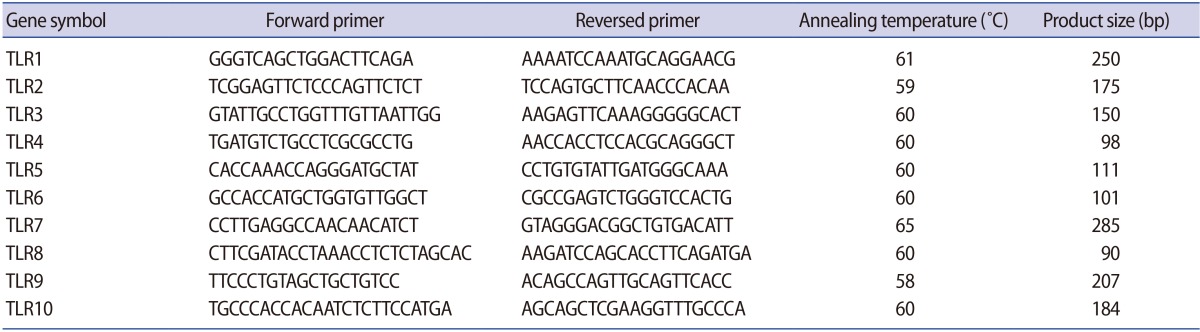
Quantitative real-time polymerase chain reaction (QPCR) was performed with the cDNA prepared from ciliated and non-ciliated epithelial cells. QPCR reactions were carried out in triplicate using an ABI Prism 7300 Sequence Detector (Applied Biosystems, Foster, CA, USA) with a total volume of 20 µL, containing 250 ng of cDNA, 5 pmol of gene-specific primers, and SYBR Green reagent (Applied Biosystems) with ROX dye as a passive control for signal intensity. The thermal cycle profile was 10 seconds at 95℃, followed by 50 cycles of 5 seconds at 95℃ and 35 seconds at 60℃. Melting curve analysis was used to determine the specificity of the PCR fragments. All melting curves yielded one peak per PCR product. The expression of the reference housekeeping gene GAPDH was also quantified for the purpose of normalization, and was tested by real-time polymerase chain reaction (RT-PCR). All experiments included non-RT controls and negative controls (no cDNA). The forward and reverse primer sequences that were used are presented in Table 1. The QPCR data were analyzed using the comparative cycle threshold method [24].
9. PAMP stimulation of fallopian tube epithelial cells
To study the functionality of TLRs in fallopian tube epithelial ciliated and non-ciliated cells, the cells were exposed to the TLR2-, TLR3-, TLR5-, TLR7-, and TLR9-specific ligands peptidoglycan, poly I:C, flagellin, loxoribine, and the CPG oligonucleotide, respectively. Phorbol 12-myristate 13-acetate (PMA; Sigma-Aldrich, No. P8139) with ionomycin (Sigma-Aldrich, No. 13909) was used as a positive control in this experiment (10 ng/mL of PMA and 500 ng/mL of ionomycin). Cells were cultured in 12-well culture plates until they were confluent. The supernatant was then replaced with 1 mL of fresh culture medium containing TLR2, TLR3, TLR5, TLR7, and TLR9 ligands, peptidoglycan from Staphylococcus aureus (10 µg/mL; catalog #tlr1-pgnsa), poly I:C (25 µg/mL; catalog #tlr1-pic), purified flagellin from Salmonella typhimurium (100 ng/mL), loxoribine (100 µM; catalog #tlr1-lox), and the CPG oligonucleotide ODN1826 (10 µM; catalog #tlr1-modn) (all Invitrogen). Cells were incubated for 24 hours and the supernatants were collected, centrifuged at 10,000×g for 5 minutes at 4℃, transferred to fresh tubes, and stored at −70℃ for an enzyme-linked immunosorbent assay (ELISA). To screen for the effects of PAMP stimulation, IL-6 and IL-8 production was analyzed by ELISA [25].
10. Enzyme-linked immunosorbent assay
The concentrations of IL-6 and IL-8 were determined in culture supernatants with commercially available IL-6 and IL-8 sandwich ELISA Duoset kits (R&D Systems, Minneapolis, MN, USA). ELISA was performed according to the manufacturer's instructions with 100 µL of cell-free supernatant. The ELISA assay had a sensitivity of 18.75 pg/mL and 8 pg/mL for IL-6 and IL-8, respectively. The sample concentrations were determined by interpreting the standard curve.
11. Statistical analysis
The results were expressed as mean±standard error of the mean. The statistical analysis was conducted using the t-test. The p-values <0.05 were considered to indicate statistical significance.
Results
1. TLR immunostaining
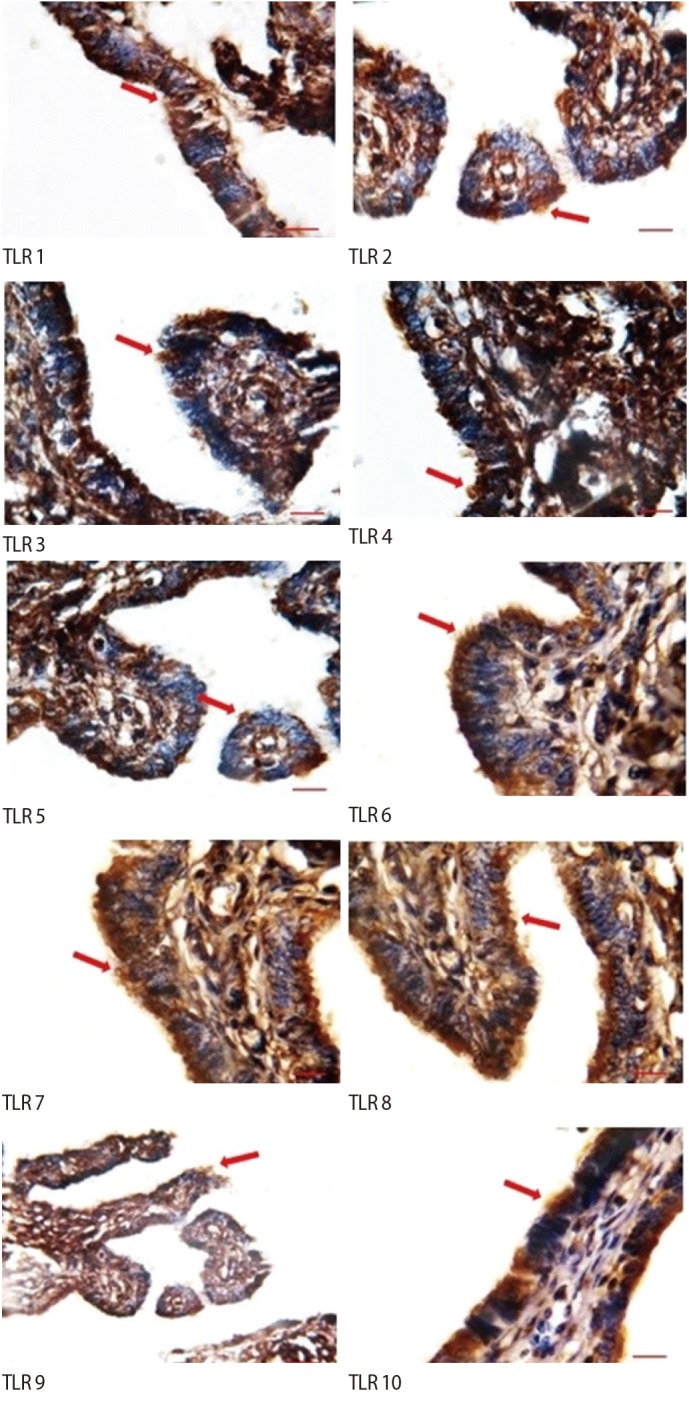
The ciliated cells had positive immunostaining for all the TLRs. However, in non-ciliated cells, weak staining was observed for TLR1–TLR8, and no staining for TLR9 or TLR10. In addition, weak staining of the stroma was observed for TLR7 and TLR9, but no staining was observed in the stroma for the other TLRs (Figure 2).
2. Cytokeratin immunostaining
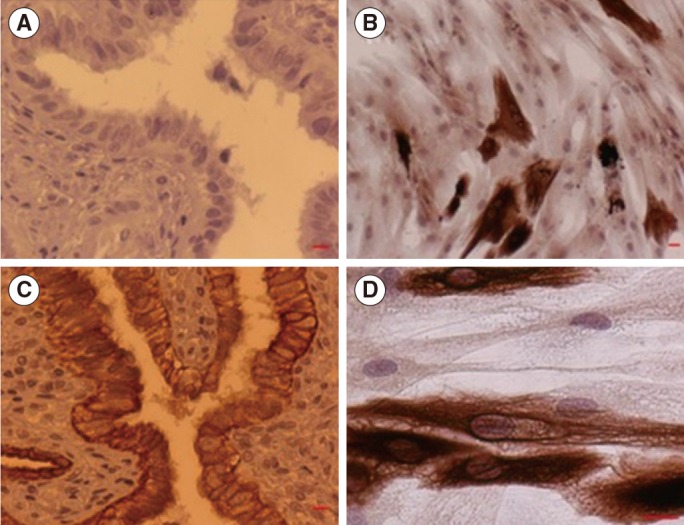
Positive cytokeratin immunostaining was observed in the epithelial layer of the fallopian tubes and in the primary cell culture (Figure 3).
3. Quantitative real-time polymerase chain reaction
The expression of TLRs 1–10 was significantly higher in the ciliated cells than in the non-ciliated cells. All amplified products were at the predicted size for their respective genes (Figure 4). Figure 5 shows the results of QPCR for the expression of the genes for TLRs 1–10 in the ciliated and non-ciliated cells.
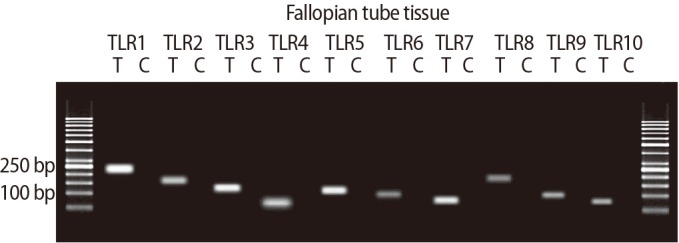
The expression of the genes for Toll-like receptors (TLRs) 1–10 in human fallopian tube tissue. Each pair of primers produced a specific product, with the specific predicted size in the test samples (T). C, control sample.
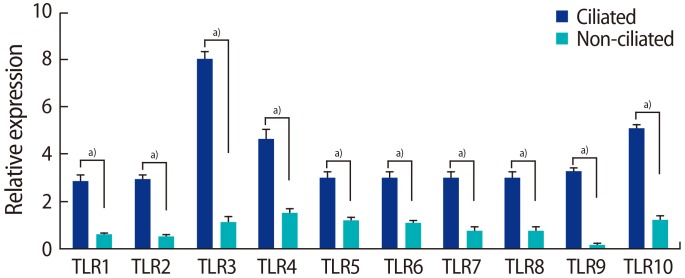
Quantitative polymerase chain reaction to assess the expression of the genes for Toll-like receptors (TLRs) 1–10 in ciliated and non-ciliated fallopian tube epithelial cells. Data are presented as mean±standard error of the normalized expression values for the genes for TLRs 1–10. Real-time polymerase chain reaction was performed three times for each comparison. a)Significant differences between groups (p<0.01).
4. TLR function in ciliated and non-ciliated cells
The production of IL-6 and IL-8 was significantly higher in the ciliated cells than in the non-ciliated cells in response to peptidoglycan, poly I:C, CPG oligonucleotide, PMA, flagellin, and loxoribine (Figures 6, 7).
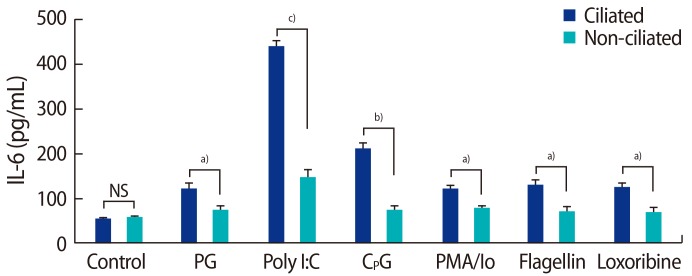
Interleukin (IL)-6 production by fallopian tube epithelial cells after 24 hours of treatment with 10 µg/mL of PG from Staphylococcus aureus, 1 µg/mL of poly I:C, 10 µM CpG oligonucleotide, 10 ng/mL of PMA and 500 ng/mL of Io, 25 µg/mL of flagellin from Escherichia coli, 1 µM loxoribine. NS, not significant; PG, peptidoglycan; PMA, phorbol 12-myristate 13-acetate; lo, Ionomycin. Significant differences between groups: a)p<0.05; b)p<0.01; c)p<0.001.
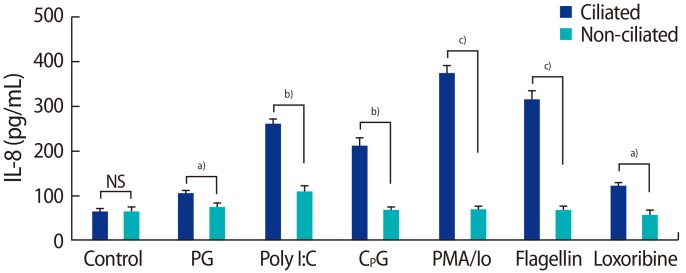
Interleukin (IL)-8 production by fallopian tube epithelial cells after 24 hours of treatment with 10 µg/mL of PG from Staphylococcus aureus, 1 µg/mL of poly I:C, 10 µM CpG oligonucleotide, 10 ng/mL of PMA and 500 ng/mL of Io, 25 µg/mL of flagellin from Escherichia coli, 1 µM loxoribine. NS, not significant; PG, peptidoglycan; PMA, phorbol 12-myristate 13-acetate; lo, Ionomycin. Significant differences between groups: a)p<0.05; b)p<0.01; c)p<0.001.
Discussion
Infection of the fallopian tubes due to chlamydia, gonorrhea, or other STIs can cause serious fertility problems. Therefore, healthy fallopian tubes are a prerequisite for a successful spontaneous pregnancy [5]. Although the upper part of the female reproductive tract was previously considered to be sterile, it has been found that pathogens in the vagina can ascend from the cervix and uterus to the fallopian tubes within minutes of deposition [26]. It has also been shown that semen can transmit viruses to the upper part of the female reproductive tract [2728]. Thus, the fallopian tubes, like other parts of the female reproductive tract, must be protected from pathogenic invasion by the immune system.
TLRs constitute the body's primary defense system because they detect and rapidly respond to micro-organisms or endogenous danger signals and initiate inflammatory cascades. Several studies have characterized the presence and the function of TLRs in the upper female reproductive tract [2930313233]. In the first part of the present study, immunostaining showed that the TLR 1–10 proteins are expressed in a cell-type-specific fashion in the fallopian tubes. We detected positive immunostaining for all the TLRs in the fallopian tube ciliated epithelial cells. However, in the non-ciliated cells, weak staining for TLRs 1–8 was observed, as well as no staining for TLRs 9 or 10.
In accordance with our results, TLRs have been shown to be expressed throughout the female reproductive tract, including the fallopian tubes, uterine endometrium, cervix, and ectocervix, as well as MYD88 and accessory molecule CD14 [34]. Another study showed that TLRs 7–9 were present in the female reproductive tract tissues, but reported that TLR10 expression was limited to the fallopian tube [35]. In contrast to our findings, a recent study failed to detect TLR10 expression in epithelial cells obtained from the fallopian tubes of premenopausal women undergoing hysterectomy [36].
In the next step of the study, based on the results of the first part, 2 cell types present in the fallopian tube were separated by the ring cloning method for further analysis. The expression of TLRs 1–10 was significantly higher in the ciliated cells than in the non-ciliated cells. Apart from the role of TLRs in immunity, they have been assumed to play an active role in many physiological and inflammatory events related to normal reproductive processes. TLRs participate in the regulation of ovulation, tissue remodeling during the menstrual cycle, sperm protection, sperm capacitation, fertilization, and pregnancy [19373839]. Shimada et al. [40] illustrated that the activation of TLR2 and TLR4 in cumulus cells in response to sperm-secreted hyaluronic acid fragments resulted in the production of cytokines and chemokines through the NF-κB pathway. The cytokines and chemokines promoted sperm capacitation through G protein-coupled receptor activation and calcium release. Additionally, our previous study showed that sperm could stimulate TLR3 and TLR5 in a physiological manner in the fallopian tube, perhaps to prepare a safe environment for the gamete and embryo [41].
Based on our findings, we speculate that ciliated cells probably participate more actively than non-ciliated cells in preparing a desirable and safe environment for gamete survival and in supporting the embryo through higher TLR expression and greater production of cytokines and chemokines. Therefore, in the next step of the study, we investigated whether the TLRs on epithelial cells were functional and whether ciliated cells produced a greater concentration of cytokines than secretory cells.
We found that ciliated epithelial cells produced more IL-6 and IL-8 in response to their ligands than non-ciliated epithelial cells. Our results agree with those of another report regarding the functional expression of TLRs 7–10 in female reproductive tract tissue cells [35]. In contrast to our findings, Ghosh et al. [36] demonstrated that fallopian tube epithelial cells were unresponsive to the TLR2 agonist zymosan. Some explanations for this difference may lie in the way the epithelial cells were prepared and the use of different agonists.
The TLR family recruits and activates several pathways, including the NF-κB, type I IFN, c-Jun N-terminal kinases (JNK), and p38 MAP kinase pathways, depending on the nature of the adaptor that is used [154243]. Stimulation of these signaling pathways leads to the production of proinflammatory cytokines and prostaglandins [15]. These factors are required for increasing the permeability of blood vessels and muscular contractions. Prostaglandins seem to play a role in the transport of gametes and embryos in the fallopian tubes. TLR-induced IL-6 and IL-8 are two key mediators in the inflammatory process that are synthesized by innate effector cells such as epithelial cells, uterine natural killer cells, monocytes, and macrophages. However, cytokine/chemokine functions are not restricted to the immune response. Compelling evidence has been reported that they play important roles in cellular differentiation, embryonic development, and the feto-maternal interface [4445]. These observations highlight the potential role of TLRs in the regulation of reproductive processes via downstream signaling, in addition to their role in protecting against invading pathogens.
Fallopian tube ciliated cells are important in tubal transport, as they allow sperm to be transported in the opposite direction to the ova and embryo, and they also support fertilization and early embryogenesis; likewise, motile human sperm have been shown to bind by their heads to the ciliated apical areas of the tubal epithelium in vitro [1]. Ciliated cells and ciliary beating can be modified by hormonal levels and impaired by infections, smoking, and gynecological disorders such as endometriosis, meaning that environmental changes also exert effects on the function of these kinds of cells [46]. Because of the vital role of ciliated cells in physiological processes and the fact that they can be influenced by environmental factors, a monitoring system such as the TLR family would seem to be crucial for them. This may explain the greater expression and responsiveness of TLRs in ciliated cells than in non-ciliated cells. Ciliated cells are also the first line of defense against pathogens [1]. It has been shown that healthy oviducts display a rapid host response to lipopolysaccharide by elevating the tubal flow and increasing the frequency of cilia movement, suggesting that cilia play an important role in detecting infections [47]. Therefore, it can be suggested that ciliated cells play a more critical role in the immune defense and reproductive processes related to the fallopian tubes than initially thought. However, more detailed studies are needed to evaluate the role of ciliated cells in the immune response and in various reproductive processes.
In conclusion, the current study demonstrated the different localization and function of TLRs within ciliated and secretory cells of the human fallopian tube. Our results indicate that the ciliated cells play a greater role than expected in the innate immune defense of the fallopian tubes. In addition, ciliated cells may play a pivotal role in preparing gametes and contributing to an embryo-supporting environment via the production of more proinflammatory cytokines. However, further studies are needed to clarify the higher expression of TLRs and their functions in ciliated cells.
Acknowledgments
The authors thank all the patients who consented to participate in this study. Further, the authors thank Mrs. Samaneh Aghajanpour for her skillful technical assistance.
Notes
This work was supported by the Royan Institute, Tehran, Iran (grant No. 90000247)
Conflict of interest: No potential conflict of interest relevant to this article was reported.