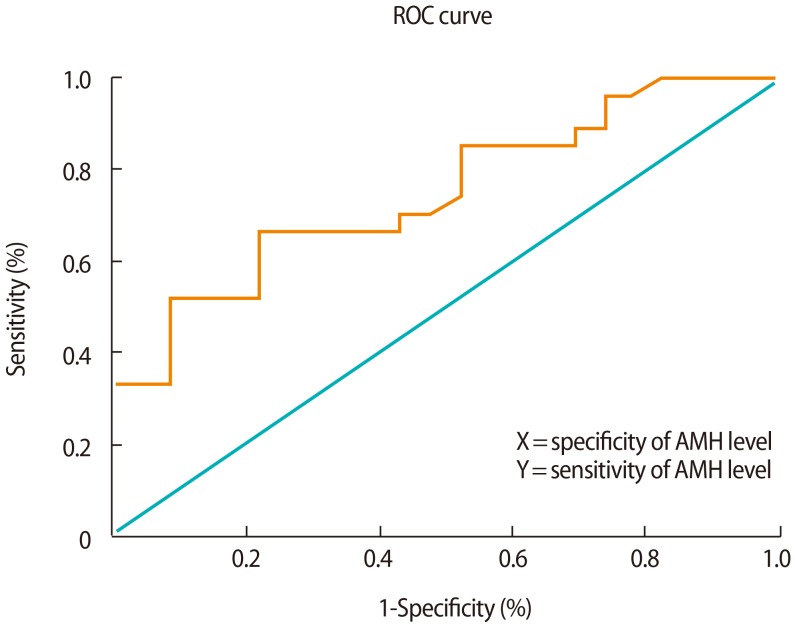
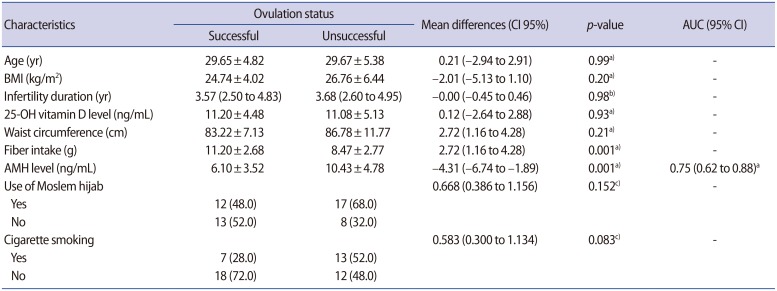
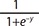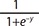1. Hart R, Norman R. Polycystic ovarian syndrome: prognosis and outcomes. Best Pract Res Clin Obstet Gynaecol 2006;20:751-778.PMID:
16766228.


2. Messinis IE. Ovulation induction: a mini review. Hum Reprod 2005;20:2688-2697.PMID:
16006478.



3. Balen A. Ovulation induction. Obstet Gynaecol Reprod Med 2004;14:261-268.

4. Ghobadi C, Nguyen TH, Lennard MS, Amer S, Rostami-Hodjegan A, Ledger WL. Evaluation of an existing nomogram for predicting the response to clomiphene citrate. Fertil Steril 2007;87:597-602.PMID:
17156783.


5. Eijkemans MJ, Imani B, Mulders AG, Habbema JD, Fauser BC. High singleton live birth rate following classical ovulation induction in normogonadotrophic anovulatory infertility (WHO 2). Hum Reprod 2003;18:2357-2362.PMID:
14585887.



6. Imani B, Eijkemans MJ, te Velde ER, Habbema JD, Fauser BC. Predictors of patients remaining anovulatory during clomiphene citrate induction of ovulation in normogonadotropic oligoamenorrheic infertility. J Clin Endocrinol Metab 1998;83:2361-2365.PMID:
9661609.


7. Kurabayashi T, Suzuki M, Fujita K, Murakawa H, Hasegawa I, Tanaka K. Prognostic factors for ovulatory response with clomiphene citrate in polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol 2006;126:201-205.PMID:
16337728.


8. Dumesic DA, Lesnick TG, Stassart JP, Ball GD, Wong A, Abbott DH. Intrafollicular antimullerian hormone levels predict follicle responsiveness to follicle-stimulating hormone (FSH) in normoandrogenic ovulatory women undergoing gonadotropin releasing-hormone analog/recombinant human FSH therapy for in vitro fertilization and embryo transfer. Fertil Steril 2009;92:217-221.PMID:
18675414.



9. Mashiach R, Amit A, Hasson J, Amzalzg S, Almog B, Ben-Yosef D, et al. Follicular fluid levels of anti-Mullerian hormone as a predictor of oocyte maturation, fertilization rate, and embryonic development in patients with polycystic ovary syndrome. Fertil Steril 2010;93:2299-2302.PMID:
19261276.


10. Lee JR, Kim SH, Kim SM, Jee BC, Ku SY, Suh CS, et al. Follicular fluid anti-Mullerian hormone and inhibin B concentrations: comparison between gonadotropin-releasing hormone (GnRH) agonist and GnRH antagonist cycles. Fertil Steril 2008;89:860-867.PMID:
18249372.


11. Catteau-Jonard S, Pigny P, Reyss AC, Decanter C, Poncelet E, Dewailly D. Changes in serum anti-Mullerian hormone level during low-dose recombinant follicular-stimulating hormone therapy for anovulation in polycystic ovary syndrome. J Clin Endocrinol Metab 2007;92:4138-4143.PMID:
17698904.



12. El-Halawaty S, Rizk A, Kamal M, Aboulhassan M, Al-Sawah H, Noah O, et al. Clinical significance of serum concentration of anti-Mullerian hormone in obese women with polycystic ovary syndrome. Reprod Biomed Online 2007;15:495-499.PMID:
18028738.


13. World Health Organization. The Asia-Pacific perspective: redefining obesity and its treatment. Sydney: Health Communications Australia; 2000.
14. Messinis IE, Milingos SD. Current and future status of ovulation induction in polycystic ovary syndrome. Hum Reprod Update 1997;3:235-253.PMID:
9322100.



15. Li L, Chen X, Mo Y, Chen Y, Wenig M, Yang D. Elevated serum antimullerian hormone in adolescent and young adult Chinese patients with polycystic ovary syndrome. Wien Klin Wochenschr 2010;122:519-524.PMID:
20809108.


16. Piltonen T, Morin-Papunen L, Koivunen R, Perheentupa A, Ruokonen A, Tapanainen JS. Serum anti-Mullerian hormone levels remain high until late reproductive age and decrease during metformin therapy in women with polycystic ovary syndrome. Hum Reprod 2005;20:1820-1826.PMID:
15802325.



17. Iliodromiti S, Kelsey TW, Anderson RA, Nelson SM. Can anti-Mullerian hormone predict the diagnosis of polycystic ovary syndrome? A systematic review and meta-analysis of extracted data. J Clin Endocrinol Metab 2013;98:3332-3340.PMID:
23775353.


18. Sahmay S, Atakul N, Aydogan B, Aydin Y, Imamoglu M, Seyisoglu H. Elevated serum levels of anti-Mullerian hormone can be introduced as a new diagnostic marker for polycystic ovary syndrome. Acta Obstet Gynecol Scand 2013;92:1369-1374.PMID:
23980726.


19. Amer SA, Li TC, Ledger WL. The value of measuring anti-Mullerian hormone in women with anovulatory polycystic ovary syndrome undergoing laparoscopic ovarian diathermy. Hum Reprod 2009;24:2760-2766.PMID:
19640893.



20. Mahran A, Abdelmeged A, El-Adawy AR, Eissa MK, Shaw RW, Amer SA. The predictive value of circulating anti-Mullerian hormone in women with polycystic ovarian syndrome receiving clomiphene citrate: a prospective observational study. J Clin Endocrinol Metab 2013;98:4170-4175.PMID:
23979947.


21. di Clemente N, Goxe B, Remy JJ, Cate RL, Josso N, Vigier B, et al. Inhibitory effect of AMH upon the expression of aromatase and LH receptors by cultured granulosa cells of rat and porcine immature ovaries. Endocrine 1994;2:553-558.
22. Das M, Gillott DJ, Saridogan E, Djahanbakhch O. Anti-Mullerian hormone is increased in follicular fluid from unstimulated ovaries in women with polycystic ovary syndrome. Hum Reprod 2008;23:2122-2126.PMID:
18550512.


24. Thomson RL, Spedding S, Buckley JD. Vitamin D in the aetiology and management of polycystic ovary syndrome. Clin Endocrinol (Oxf) 2012;77:343-350.PMID:
22574874.


25. Crosignani PG, Colombo M, Vegetti W, Somigliana E, Gessati A, Ragni G. Overweight and obese anovulatory patients with polycystic ovaries: parallel improvements in anthropometric indices, ovarian physiology and fertility rate induced by diet. Hum Reprod 2003;18:1928-1932.PMID:
12923151.