Application of two different synthetic sequential media for the human IVF-ET program: a prospective, randomized, and comparative study
Article information
Abstract
Objective
Since IVF program was first established, various types of media and culture systems have been developed either in-house or commercially. The aim of this study was to compare the efficacy of in-house Maria Research Center (MRC) media to that of commercially available Sydney IVF media in human day 3 embryo transfer cycles.
Methods
Three hundred sixty nine couples were included in this prospective, randomized, and comparative study. All couples undergoing IVF treatment at the Maria Fertility Hospital were randomly assigned to either Sydney IVF (n=178) or MRC (n=191) media.
Results
No difference was observed between the MRC media and Sydney IVF media groups with respect to fertilization rate (74.4% vs. 75.5%). The clinical pregnancy and implantation rates of MRC media (47.1% and 20.0%, respectively) were also similar to those of Sydney IVF media (44.4% and 19.4%, respectively). However, the proportion of embryos with good quality on day 3 was significantly higher in the MRC media group than the Sydney IVF media group (50.2% vs. 43.2%) (p<0.05).
Conclusion
MRC media were as effective as Sydney IVF media for sustaining embryo development and pregnancy rates. The present study implies that MRC media can be a suitable alternative to commercially available media for human IVF-ET program.
Introduction
Since IVF program was first established, a large number of media and culture systems have been developed. Maria Fertility Hospital has used YS medium for controlled ovarian hyperstimulation and IVM cycles of human IVF [1-4]. YS medium has been shown to support the development of both oocytes and zygotes to the blastocyst stage in co-culture systems composed of autologous cumulus cells and human follicular fluid. This culture system also achieved an acceptable pregnancy rate in a human IVF-ET program [1,2]. However, there are potential disadvantages associated with this system, including quality control difficulties and being time-consuming and labor intensive [5-7]
To avoid those problems, quality-controlled commercial media have been used in many IVF laboratories [7]. Nevertheless, commercial media are costly and difficult to compare directly because their exact compositions are not clearly indicated [8-10]. Furthermore, clinical outcomes of commercially available sequential media have been reported to be in the same range as those obtained using a co-culture system [11-14].
Accordingly, the use of in-house synthetic sequential media would be desirable in a human IVF-ET program to avoid disadvantages associated with co-culture systems and commercial media and to obtain a high pregnancy rate. Consequently, a synthetic sequential media known as Maria Research Center (MRC) media were newly developed after modifying the YS medium. The present study was conducted to compare the efficacy of MRC media in the human IVF-ET program to that of commercially available media.
Methods
1. Inclusion criteria
The present study was conducted following approval by the Institutional Board of the Maria Fertility Hospital. This study included women younger than 40 years old who visited Maria Fertility Hospital at Seoul from November 2009 to September 2010. Ovarian stimulation was induced by GnRH agonist long treatment or GnRH antagonist short treatment.
Patients were randomly assigned to one of two media, Sydney IVF media as a control group and MRC media as a trial group (Table 1), at oocytes retrieval. A series of Sydney IVF media consisting of Sydney IVF fertilization medium (COOK, Brisbane, Australia), Sydney IVF cleavage medium (COOK) and Sydney IVF blastocyst medium (COOK) was used at our discretion as a representative of commercially available media. Those patients who showed either fertilization failure in both two groups or less than two 2 pronuclear embryos in either of the two groups were excluded.
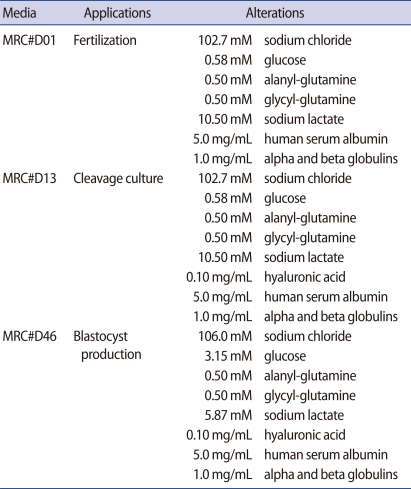
2. Preparation of MRC media
A series of MRC media consisting of MRC#D01 for fertilization, MRC#D13 for cleavage growth and MRC#D46 for blastocyst growth was newly tailored by modifying the YS medium. All MRC media were designed to meet the nutritional requirements specific to embryonic stages (Table 1) [15-20]. Each medium was prepared using sterile water for injection in a class 1,000 clean room and then examined to ensure that it met the standard manufacturing specifications, including quality control tests using a mouse embryo assay (MEA), pH measurement, osmolarity tests, and endotoxin assays. All MRC media had similar parameters of osmolarity and pH in a 6% CO2 atmosphere at 37℃ (278 to 280 mOsm/kg and 7.30 to 7.45, respectively).
3. Application of MRC media to human IVF-ET cycles
Oocytes retrieval was conducted approximately 36-38 hours after hCG injection by transvaginal ultrasound guidance. Oocytes of the control group were cultured in Sydney IVF fertilization medium, while those of the trial group were incubated in MRC#D01 medium. Fertilization was induced by either conventional IVF or ICSI approximately 4-6 hours after oocytes retrieval and confirmed approximately 17-19 hours after insemination. Zygotes in the control group were cultured in Sydney IVF cleavage medium, whereas those of the trial group were cultured in MRC#D13 medium. The culture conditions were 40 µL droplets in a 6% CO2, 5% O2, and 89% N2 incubator at 37℃. Embryo development was monitored daily and embryo grades were assessed based on the number of blastomeres and the amount of cellular fragmentation. On day 3, the embryos were classified into four grades: embryos which reached the 6-cell or more stage and had no cytoplasmic fragmentation (grade 1); embryos which reached 6-cell or more stage but less than 20% cytoplasmic fragmentation (grade 2); embryos which developed 6-cell or more stage but had 20-50% cytoplasmic fragmentation or embryos which did not reach the 6-cell stage but had less than 50% cytoplasmic fragmentation (grade 3); and embryos which did not reach 6-cell and had more than 50% cytoplasmic fragmentation (grade 4). Grade 1 and grade 2 were regarded as good-quality. The mean and SD of the embryo quality were calculated for each media group among individual patients. Those embryos with the highest scores from individual patients were transferred on day 3 using an embryo transfer catheter (COOK).
Supernumerary embryos in the control group were cultured in Sydney IVF blastocyst medium, while those in the trial group were cultured in MRC#D46 medium. Embryos that reached the expanded blastocyst stage were vitrified and cryopreserved in liquid nitrogen [21].
Clinical pregnancy was assessed by the presence of a gestational sac and fetal heart beat at 5-6 weeks after the embryo transfer. Ongoing pregnancy was followed up by the presence of a fetal heart beat 10 weeks after embryo transfer.
4. Statistical analyses
Statistical analyses were conducted using the χ2 test to compare the embryo qualities, clinical and ongoing pregnancy rates, and implantation rates of the two groups. A t-test was used to compare the number of retrieved oocytes, normal zygotes, and transferred embryos. Data were analyzed with SPSS (SPSS Inc., Chicago, IL, USA) and a p-value less than 0.05 was considered statistically significant.
Results
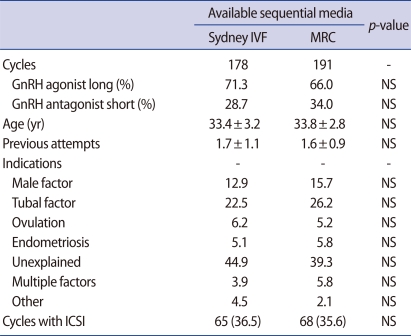
A total of 369 human IVF-ET cycles were assessed in the present study. Oocytes and zygotes of 178 cycles were sequentially cultured in Sydney IVF media as a control group, while those of 191 cycles were sequentially incubated in MRC media as a trial group. Several characteristics of patients are presented in Table 2. Patient age, infertility indications, and previous IVF attempts did not different between groups. In addition, there was no difference in the proportion of GnRH agonist administrations (71.3% vs. 66.0%) and ICSI trials (36.5% vs. 35.6%) observed between groups (Table 2).
The number of oocytes collected from the control group was similar to that collected from the trial group (10.8±5.8 vs. 10.6±6.0) (Table 3). In addition, there was no difference in the number of metaphase II stage oocytes between the control and trial groups at retrieval (8.5±4.7 vs. 8.4±4.8). The fertilization rate of the trial group was also similar to that of the control group (74.4% vs. 75.5%). However, the proportion of embryos with good quality on day 3 was significantly higher in the trial group than the control group (50.2% vs. 43.2%) (p<0.05). The proportion of transferred embryos with good quality was also higher in the trial group than in the control group (78.8% vs. 70.1%) (p<0.05). The qualities of day 3 embryos were not significantly influenced by inter-batch variations in either group (Figure 1).
The number of cycles with surplus embryos was 159 cycles for the control group and 142 cycles for the trial group (Table 3). When the embryos that reached the expanded blastocyst stage were vitrified and cryopreserved, differences in the proportion of cycles with embryo cryopreservation were not observed between the control and trial groups (34.0% vs. 36.6%) (Table 3).
The clinical and ongoing pregnancy rates of the trial group (47.1% and 39.3%, respectively) were similar to those of the control group (44.4% and 37.1%, respectively) (Table 3). Similarly, the implantation rate of the trial group was identical to that of the control group (20.0% vs. 19.4%) (Table 3).
Discussion
YS medium containing hFF has been shown to support fertilization of human oocytes and development of human zygotes to the blastocyst stage on a cumulus cell monolayer, resulting in an acceptable pregnancy rate [1-4]. However, several reports claimed that the use of maternal serum and co-culture with feeder cells was associated with high rates of fetal loss, potential for sample identification error, and possible induction of embryo-toxic factors into the IVF media [11,13,14]. Therefore, Maria Fertility Hospital has newly developed sequential culture media by modifying the YS medium that were specialized for fertilization (MRC#D01), cleavage (MRC#D13), and blastocyst growth (MRC#D46) (Table 1). The present comparative and randomized study was conducted to compare the efficacy of the series of MRC media to that of commercially available media in a human day 3 embryo transfer program.
A number of studies have been conducted to compare the effectiveness of commercially available media types for embryo growth and pregnancy rates. Mauri et al. [7] compared P1 medium to IVF-50 medium, while Zollner et al. [22] compared G1.2/G2.2 media to M1/M2 media. They concluded that there were no significant differences in the pregnancy and implantation rates between the two types of media. However, Van Langendonckt et al. [23] reported that the G1.2/G2.2 media group had higher pregnancy and implantation rates than the Sydney IVF media group, especially in a group of patients having at least five zygotes. Ben-Yosef et al. [24] compared P1 media to Sydney IVF media and found that the P1 Media appeared to be associated with a higher embryo cleavage rate and improved implantation rates. Recently, Sifer et al. [25] reported that GIII series significantly improved day 2 and day 3 embryo qualities when compared to ISM1/2 media, along with higher rates in clinical pregnancy and implantation. Collectively, these previous reports indicate that each type of media has its unique distinct properties over other media types during specific stages of the human IVF-ET program and that each type of media results in various outcomes depending on the different IVF-ET program regimes.
Thus, Maria Fertility Hospital conducted randomized preliminary studies to select a control media group among commercially available P1, G1.5/2.5, and Sydney IVF media (data not shown). The embryo quality and clinical pregnancy rate were higher in the Sydney IVF media group than in other media groups, even though previous studies revealed that embryos cultured in Sydney IVF media displayed lower pregnancy rates than G1.2/2.2 or P1 media [23,24]. As a result, the series of Sydney IVF media was used as a representative commercially available media (control group) for comparison with the series of MRC media (trial group).
All variables, such as patient ages, infertility indications, previous IVF attempts, and ICSI cycle ratio, did not differ between the control and trial groups (Table 2). The rates of implantation, clinical pregnancy, and ongoing pregnancy in the trial group were identical to those in the control group. The only difference between groups was the quality of day 3 embryos. Specifically, the embryos cultured in the series of MRC media had a better morphological quality and showed a lower fragmentation rate than those cultured in the series of Sydney IVF media (p<0.05), resulting in a higher quality of transferred embryos in the series of MRC media than the series of Sydney IVF media (78.8% vs. 70.1%) (p<0.05). After transferring a maximum of three good quality embryos per cycle, the supernumerary embryos were sequentially cultured in each group. The embryonic development to the blastocyst stage was equal in both groups and the number of cycles with blastocyst cryopreservation did not differ significantly between the control and trial groups (34.0% vs. 36.6%) (Table 3). Although embryonic development was superior in the MRC media group on day 3, pregnancy rates and the number of cryopreservation cycles were nearly the same in both groups. This observation was similar to the results of a previous study [23] in which embryos cultured in Sydney IVF media had a better morphological aspect, but showed a lower implantation rate than those obtained in the series of G media (Vitrolife). These results suggest that embryo morphology is not a reliable marker for comparing different types of culture media for pregnancy rates. In this regard, several studies have demonstrated that traditional criteria for embryo selection on day 3 for ET may be limited by the fact that the embryos are still partially dependent on the maternal genome [26,27]. The embryonic genome is fully activated after the 8-cell stage, implying that the blastocyst formation on day 5 offers more information regarding the implantation potential [26-29]. Consequently, additional randomized studies of blastocyst formation or blastocyst transfer outcomes using the series of MRC media are necessary.
Early embryonic development can be differentially controlled by varying the nutritional composition of the culture media. However, it is difficult to identify what is responsible for better embryo quality since precise nutritional compositions of most culture media are not publicly known. Based on the technical bulletin of Sydney IVF media, there are two major differences between Sydney IVF media and MRC media. The first difference is the presence of alpha- and beta-globulin in MRC media. Several studies have reported that the use of synthetic serum substitute containing alpha- and beta-globulin has beneficial effects during the culture of human and mouse embryos [18,20,30]. The second difference is the use of hyaluronic acid in MRC media, which is found at high concentrations in the fluid of the female reproductive tract [31-33]. However, the rates of clinical pregnancy and implantation were similar between both groups, even though MRC media had a better morphological aspect than Sydney IVF media. Macklon et al. [34] and Van den Bergh et al. [35] insisted that when producing and managing a culture system, quality more important to successful human embryo culture than the type of media, indicating the importance of the establishment of a standard method for the quality control of media. Maria Fertility Hospital established standardized quality control procedures, such as MEA, osmolarity-and pH-controls, and endotoxin testing, and applied them to screen embryo toxicity in chemicals and media. Gardner et al. [36] reported that the results of quality control are dependent on the strain of mice used because inbred strains and their F1 hybrids are less sensitive to their environment than outbred strains of mice. In the present study, B6D2 F1 mice for MEA were used because the CF-1 outbred mice were not commercially available on a regular basis within Korea. However, it is thought that B6D2 F1 mice embryos are suitable for MEA because good quality embryos and a high pregnancy rate were obtained in human IVF using media screened with these embryos. Nevertheless, further studies comparing the effectiveness between B6D2 F1 mice and other outbred mice are needed to establish a critical quality control method.
Although commercially available media have commonly been used in most IVF programs worldwide, there are several advantages to the use of in-house synthetic sequential media at IVF laboratories. First, the results of quality control should be reliable due to a stringent protocol setting-up. Second, any direct supplementations or deletions of specific components are feasible to obtain good quality embryos and high pregnancy rates since those components can be defined. Finally, dependence on commercially available media diminishes so that the laboratory can more easily accommodate unexpected situations or problems, such as a sudden increase in IVF patients. Despite these advantages, inter-batch variations of in-house media have been reported to be a major problem in clinical settings [5-7]. Therefore, data obtained in this study were analyzed to determine if inter-batch variations influenced embryo quality. There were eight batches used that had over 10 IVF cycles per batch in each group of the present study. The results showed that the quality of day 3 embryos was not affected by inter-batch variations in either group (Figure 1), which was consistent with the results of a previous study conducted by Aoki et al. [37]. However, additional studies should be conducted to elucidate the possible effects of inter-batch variation on implantation and pregnancy rates.
In conclusion, MRC media were developed by alteration in YS medium in accordance with stage-specific requirements based on the nutritional components of oviduct and uterine fluids. It would be desirable to avoid the disadvantages of serum and commercial media. The present randomized study showed that there are no significant differences between MRC media and Sydney IVF media regarding pregnancy, implantation rates, and the proportion of cycles with cryopreservation. Therefore, the results of the present study suggest that the series of MRC media can be a suitable alternative to commercially available media for human IVF-ET programs.
Notes
No potential conflict of interest relevant to this article was reported.