|
 |
- Search
| Clin Exp Reprod Med > Epub ahead of print |
Abstract
Objective
Endometriosis is a common gynecological disease among reproductive-age women. Numerous hypotheses exist regarding the pathogenesis of endometriosis. In Turkey, the consumption of Allium cepa (commonly known as the “onion cure”) is a popular treatment employed to alleviate a variety of gynecological disorders.
Methods
In this study, our objective was to assess the therapeutic mechanisms of the onion bulb A. cepa using an autologous endometriosis model in Sprague-Dawley rats. Previous research has shown that A. cepa possesses anti-inflammatory, antioxidant, and antiapoptotic properties. We evaluated the pathological condition of endometriotic implants by employing hematoxylin-eosin staining and Ki67 immunohistochemistry analysis. Transforming growth factor-beta 1 (TGF-β1) and alpha-smooth muscle actin (α-SMA) have been identified as profibrotic markers that are highly overexpressed in endometriotic tissues relative to eutopic endometrial tissue. Furthermore, TGF-β1 influences the differentiation and progression of endometriosis. To quantify profibrotic activity, we measured TGF-β1 and α-SMA using the immunosorbent assay method.
Results
Lower histologic evaluation scores for endometriotic implants were observed in the group receiving high-dose A. cepa relative to the other groups. Ki67 expression was reduced following the high-dose A. cepa regimen, which consisted of 30% A. cepa and 70% normal feed. However, no statistically significant differences in TGF-β1 or α-SMA levels were observed among the groups (p=0.7 and p=0.778, respectively).
Endometriosis is a common inflammatory gynecological pathology characterized by the ectopic implantation of endometrial tissues [1]. Endometriosis can damage the tissue through inflammatory, proliferative, and fibrogenic processes. This condition is estimated to affect nearly 10% of the general female population and between 25% and 40% of women who are infertile [2]. Furthermore, endometriosis is present in approximately 70% of women with chronic pelvic pain [3]. The primary clinical manifestations of endometriosis include chronic pelvic pain, dysmenorrhea, dyspareunia, and infertility.
The pathogenesis of endometriosis is multifactorial and continues to provoke debate concerning its pathophysiology and treatment strategies. A variety of theories contribute to our understanding of endometriosis, including the roles of inflammatory mediators, infectious agents, endocrine factors, and proangiogenic elements. Recent studies have focused on the inflammatory and immune mechanisms underlying endometriosis. Increased oxidative damage, as evidenced by elevated levels of malondialdehyde, superoxide anions, and hydrogen peroxide, has also been implicated in the disease process [4]. Recommended treatments encompass medical therapies, surgical interventions, herbal remedies, and specific anti-inflammatory and antioxidant agents.
Fibrosis refers to a loss of tissue function due to repetitive damage. This condition is marked by increased activity of myofibroblasts and heightened collagen production, causing the primary tissue to lose its functionality [5]. Histologically, fibrosis in endometriosis is evidenced by the envelopment of endometrial glands and stroma by dense fibrous tissue [6]. Furthermore, fibrosis contributes to the degradation of both the vasculature and tissue functions, potentially leading to resistance against medical treatments and hormonal suppressive therapies [7]. Transforming growth factor-beta 1 (TGF-β1) and alpha-smooth muscle actin (α-SMA) have been identified as key profibrogenic biomarkers that can be used to assess myofibroblast activity and the extent of fibrosis in endometriotic tissue [8]. Ki67, a nuclear protein, is intimately associated with cell division, as it is present during all active phases of the cell cycle (G1, S, G2, and mitosis) but is conspicuously absent in the resting phase, G0 [9]. Ki67 is a crucial marker for determining a cell’s proliferative capacity. Therefore, due to its role in signaling proliferative activity in neoplastic diseases, an increased level of Ki67 is considered a key factor for predicting the prognosis and the risk of recurrence in endometriosis cases [10].
Allium cepa, commonly known as the onion bulb, is a member of the Amaryllidaceae family [11]. Prior research has explored its anticancer and antioxidant properties [12]. Studies have shown that A. cepa possesses anti-inflammatory effects, as evidenced by reduced levels of prostaglandin E2, nuclear factor kappa-light-chain-enhancer of activated B cells, and thromboxanes, as well as the inhibition of chemotaxis [13]. Furthermore, A. cepa has been reported to ameliorate cellular degeneration and mitigate damage caused by ischemia and reperfusion in ovarian tissue [14]. Quercetin, flavonoids, and organosulfur compounds have been identified as the primary active components in A. cepa that contribute to its anti-inflammatory effects. The literature also describes the inhibition of endometriotic proliferation through the regulation of the cyclin pathway by quercetin molecules [15]. The objective of the present study is to investigate whether A. cepa can improve the histological parameters of endometriotic lesion proliferation and the levels of profibrotic mediators.
This study received approval from the Dokuz Eylül University Laboratory Animals Local Ethics Committee (Approval No: 41-2020). Twenty-one adult Sprague-Dawley rats, each weighing between 220 and 250 g, were acquired from the Dokuz Eylül University Experimental Animal Laboratory. The trial was conducted in accordance with the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines. The rats were housed in standard cages with sawdust bedding, maintained at a room temperature of 22±2 °C, and kept under a 12/12-hour dark/light cycle throughout the trial. To determine the estrus phase of the rats, vaginal smears were collected four times daily. Each procedure was documented using a digital camera. The 21 rats were randomly divided into three groups of seven animals each. Surgical procedures were carried out under sterile conditions. Anesthesia was induced with an intraperitoneal injection of ketamine hydrochloride (50 mg/kg, Ketalar; Pfizer Inc.) and xylazine hydrochloride (7 mg/kg, Alfazyne; Alfasan International BV). All laboratory procedures are detailed in Figure 1.
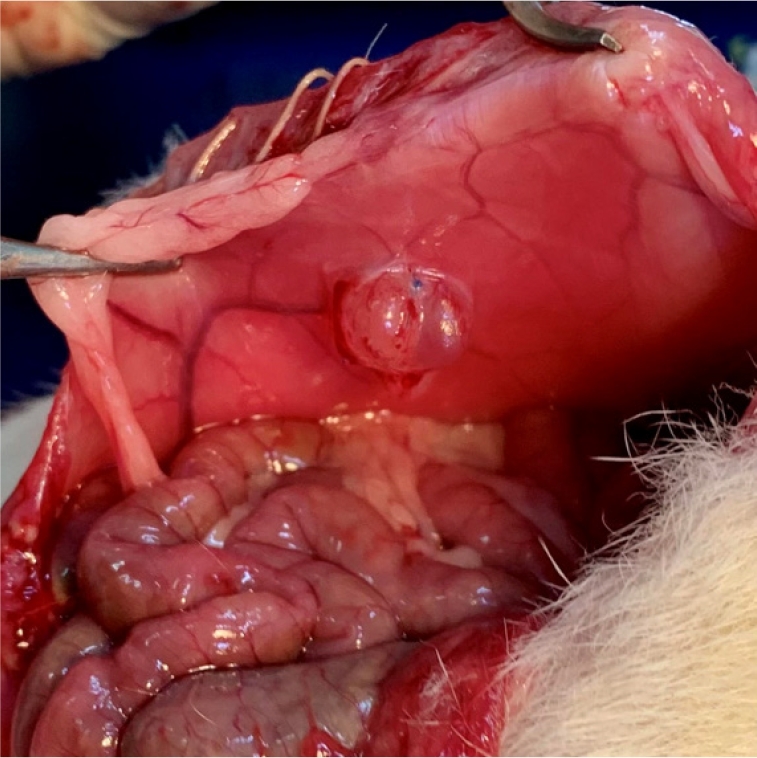
Before the incision was made, the abdominal wall was shaved and cleansed with a 10% povidone-iodine solution. With the rat under anesthesia, a sterile incision measuring 2 cm was made along the midline of the lower abdomen. The uterine horns, adnexa, and ovaries were identified in each animal. Autologous uterine tissue (1 cm long) was harvested from the distal end of the right uterine horn. This tissue was then immersed in a phosphate-buffered saline solution for 2 minutes. Subsequently, the endometrium was isolated, and endometrial fragments measuring 5×5 mm were implanted into the abdominal wall (Figure 2).
The abdominal wall and skin were closed using 3.0 polyglactin sutures. Following sterilization of the abdominal wall with 10% povidone-iodine, heating pads were applied until the animal regained consciousness. To induce the formation of endometriotic implants in autologous tissues, 50 μg/kg of estrogen was administered subcutaneously to the rats twice weekly [16].
Relaparotomy was scheduled for day 14 to validate the presence of endometriotic implants on the inner abdominal wall, coinciding with the estrus phase in rats. The anesthetic and surgical protocols from step 1 were repeated, from the initial incision to the closure of the abdominal cavity. To determine the estrus phase, daily vaginal smears were conducted. Three rats were excluded from the remainder of the study due to the inadequate development of endometriotic lesions.
Feed fortified with A. cepa was incorporated into the rats’ regular diet. The A. cepa onion bulbs were peeled, sliced, and air-dried for 1 week before being ground into a fine powder. Group A (n=6), designated as the Sham group, was administered only a 0.9% sodium chloride solution via oral gavage along with their standard diet. Group B (n=6), the low-dose group, received a mixture of 10% A. cepa and 90% standard feed, while group C (n=6), the high-dose group, was given a blend of 30% A. cepa and 70% standard feed through oral gavage daily for 21 days. The A. cepa-supplemented diet was initiated after the stabilization period following relaparotomy.
Under anesthesia, the rats were euthanized, and their endometriotic implants were harvested for both histopathological and biochemical assessments. Concurrently, blood samples were obtained for biochemical analysis. The collected endometriotic implants were subjected to histological and biochemical examinations. For histological evaluation, the tissues were preserved in 10% formalin.
Endometriotic implants were embedded in paraffin blocks following formalin fixation. Tissue sections, 5 mm thick, were prepared, then stained with hematoxylin-eosin and examined under light microscopy (CX-41; Olympus). Blinded histological assessment was conducted by a histologist with experience in a prior study of rat endometriosis, and the findings were documented photographically [17,18].
The epithelial lining of the endometrial implants underwent semi-quantitative evaluation based on a method previously outlined in the literature [17,18]. The grading was as follows: grade 0 indicated an absence of epithelium, with a corresponding score of 0; grade 1 represented a poorly preserved epithelium, characterized by the presence of only occasional epithelial cells, and was assigned a score of 1; grade 2 denoted a moderately preserved epithelium accompanied by leukocyte infiltration, with a score of 2; and grade 3 described a well-preserved epithelial lining, which received a score of 3.
Whole blood was drawn into blood collection tubes without anticoagulant and then allowed to clot naturally at 25 °C for 30 minutes. Following coagulation, the samples were centrifuged at 2,000 ×g and 4 °C for 15 minutes. The upper layer of yellow serum was then carefully collected and stored at −80 °C. Upon thawing, the serum concentrations of TGF-β1 and α-SMA were measured using an enzyme-linked immunosorbent assay kit (BTLAB, catalog numbers E1688Ra and E2330Ra). All procedures were performed in strict accordance with the instructions provided with the kit.
Sequential 5-μm-thick sections were cut from the paraffin blocks. For antigen retrieval, these sections were boiled for 9 minutes in 0.01 mol/L sodium citrate buffer at a pH of 6. To suppress endogenous peroxidase activity, the sections were then incubated in a 3% hydrogen peroxide solution for 10 minutes. Immunohistochemical staining was carried out using the avidin-biotin immunoperoxidase method, employing the Ki67 rabbit polyclonal antibody (catalog no. NB500-170SS; Novus Biologicals) as the primary antibody.
Statistical analysis of the data gathered during the study was conducted using SPSS ver. 26.0 (IBM Corp.). Means and standard deviations were calculated for the evaluation, with biochemical and parametric data presented as mean±standard deviation. Differences among groups were assessed using the Kruskal-Wallis test, and the source of any differences was further investigated with the Mann-Whitney U test. A p-value of less than 0.05 was considered indicative of statistical significance.
Throughout the trial, we observed no adverse effects—such as hair loss, fatigue, or loss of appetite—in rats fed A. cepa. All the animals survived until the conclusion of the study, and none exhibited signs of wound infection.
Figure 3 displays representative histopathological images of endometriotic implants. Table 1 presents the mean scores from the histopathological evaluation of the implants following the treatment period. A significant reduction in the mean histopathological evaluation score was observed for group C (1.14±1) relative to groups A and B (2.71±0.7 vs. 2.28±1.2, respectively; p<0.05).
The levels of tissue biomarkers (TGF-β1 and α-SMA) were assessed across groups. The data for TGF-β1 and α-SMA levels are presented in Table 2. Notably, we observed no statistically significant difference in TGF-β1 levels among groups A, B, and C (633.21±89.49 vs. 697.66±76.53 vs. 636.08±156.11, p=0.7). Similarly, α-SMA levels exhibited no significant changes following treatment with A. cepa in groups A, B, and C (36.26±6.81 vs. 32.84±5.75 vs. 33.98±8.90; p=0.778). Immunohistochemical analysis revealed that Ki67, a marker of cell proliferation, was highly expressed in all areas of the endometriotic implantation tissues in group A and in the low-dose A. cepa group (group B). However, Ki67 expression was diminished in group C, which received a high-dose of A. cepa (30% A. cepa+70% normal feed), as depicted in Figure 4.
In this study, we demonstrated that high-dose A. cepa intake significantly reduced histological evaluation scores and decreased Ki67 expression in a surgically induced rat model. However, the levels of the profibrotic mediators TGF-β1 and α-SMA did not change significantly following A. cepa administration.
Endometriosis is a common gynecological disorder characterized by chronic pelvic pain, infertility, pelvic organ dysfunction, and impairment in daily activities and overall quality of life. The clinical manifestations of endometriosis are driven by chronic inflammation, which is evidenced by elevated local and systemic levels of inflammatory chemokines and cytokines [19]. These mediators play key roles in histopathological cell damage and the clinical symptoms observed. Despite this, the underlying mechanisms of endometriosis remain incompletely understood, and definitive treatment protocols are a subject of ongoing research. Inflammatory mediators such as tumor necrosis factor alpha, interleukin 1β (IL-1β), IL-6, IL-12, prostaglandin E2, thromboxane, and others are also involved in the pathophysiology of endometriosis [20].
The progression of this condition is similarly impacted by inflammatory mediators, cytokines, and angiogenic and fibrogenic factors. The regulation of these mediators may reduce the severity of endometriosis and alleviate gynecological symptoms. Strategies that target antiproliferative, proapoptotic, autophagic, anti-cell migration and invasion, antifibrotic, and anti-angiogenic mechanisms have been explored to inhibit the progression of endometriosis [21]. Furthermore, modulators of the immune system and inhibitors of angiogenesis have been studied as alternatives to hormonal therapy and nonsteroidal anti-inflammatory drugs (NSAIDs) for the improvement of endometriosis-related clinical outcomes [22].
Deep endometriosis is the primary predisposing factor for poor outcomes. It is characterized by a rigid and compromised pelvic structure resulting from extensive fibrosis and the activation of myofibroblasts [23]. The recurrent tissue injury and repair (ReTIAR) theory is among the most prevalent explanations for deep endometriosis and the associated fibrosis. The key mechanisms of ReTIAR include overexpression of TGF-β1, THY-1, and peroxisome proliferator-activated receptor gamma; the transformation of fibroblasts into myofibroblasts; and recurrent tissue injury [24].
In the present study, hematoxylin-eosin staining revealed a morphological enhancement in group C (which received a high-dose A. cepa regimen) relative to the other groups. Observations of epithelial integrity, inflammation, glandular structures, and vasculature in endometriosis tissue were less frequent in group C. These findings corroborate a significant decrease in histological features associated with endometriosis following the intake of A. cepa.
A. cepa contains a variety of chemical constituents, including flavonoids (such as quercetin), lipophilic antioxidants, and isoliquiritigenin (ISL) [25]. These components are associated with a range of therapeutic benefits, including analgesic, antitumor, antihyperlipidemic, and antithrombotic effects [17]. Two studies have been conducted on the impact of A. cepa on endometriosis [15,26]. The findings from these studies indicate that quercetin and ISL not only improve endometriosis-associated clinical symptoms and lesions but also may influence antiproliferative and anti-inflammatory agents within endometriotic tissue. Additionally, ISL exhibits a proapoptotic effect on endometriotic cells. These results align with prior research that has documented histological improvements in endometriotic tissue.
The presence of fibrosis within endometriotic tissue can lead to anatomical distortion in the pelvis and chronic pelvic pain. Furthermore, TGF-β not only plays a critical role in fibrosis but also influences apoptosis, disease progression, and cellular differentiation in endometriosis [27]. Myofibroblast-like cells that are positive for α-SMA have been observed within fibrotic areas in ovarian, deep, and superficial endometriotic implants. One study noted markedly elevated levels of fibrotic mediators, such as α-SMA and fibronectin, in endometriotic lesions relative to the eutopic endometrium [28]. Consequently, TGF-β and α-SMA, along with associated profibrotic signaling pathways, may serve as potential therapeutic targets for the treatment of endometriosis.
In the literature, two studies have investigated the levels of TGF-β1, α-SMA, and fibrosis in rats with endometriosis treated with various substances [23,29]. These studies suggest that targeting the antifibrotic mechanism could be considered as an alternative treatment approach for endometriosis. However, we did not observe significant changes in the levels of the profibrotic mediators TGF-β and α-SMA in our surgically induced endometriosis rat model.
Previous research has indicated that the activation of the fibrinogen pathway by fibrotic mediators in endometriosis leads to reduced effectiveness of cyclooxygenase (COX) inhibitors. This reduction in drug efficacy is attributed to lower levels of prostaglandin E2 and diminished expression of COX-2 [30]. Another study demonstrated that A. cepa exhibits anti-inflammatory and analgesic effects relative to indomethacin [31]. That research indicated that A. cepa is associated with a significantly shorter pain reaction time on pain stimulation tests compared to indomethacin. The findings suggest that the diminished analgesic efficacy of NSAIDs in the presence of A. cepa within endometriotic lesions could be a consequence of fibrosis.
Ki67, which can be detected through immunohistochemistry, serves as a marker of cellular proliferation. This enables the assessment of activity levels within endometriotic lesions, providing valuable insights. An increase in Ki67 expression suggests that cells are becoming autonomous and may exert an influence on adjacent tissues [32]. Such an increase may be linked to the aggressiveness of endometriosis. Previous research has suggested that an increase in cell number may indicate uncontrolled cell division, and a correlation has been found between the severity of endometriosis and Ki67 expression levels [33]. In our study, a high-dose A. cepa regimen (comprising 30% A. cepa and 70% standard feed) resulted in a reduction of Ki67 expression compared to that observed in the other groups.
Moreover, elevated Ki67 expression has been more commonly observed in infertile women, with a strong correlation between Ki67 levels and postoperative pregnancy. Notably, 66.7% of patients with high Ki67 expression have been found to achieve pregnancy within 1 year after endometriosis surgery, underscoring the importance of endometrial resection in the treatment of infertility [34]. Our findings suggest that treatment with A. cepa may reduce Ki67 expression in lesions, which could be advantageous for infertile patients and avoid the need for surgical intervention during the follow-up period.
In conclusion, A. cepa may enhance histological outcomes as reflected by evaluation scores and reduce Ki67 expression when administered in high doses. However, no statistically significant change was detected in the levels of profibrotic mediators. A. cepa appears to exert a more pronounced effect on the proliferation aspect of endometriosis rather than on antifibrotic characteristics.
Figure 1.
Explanation of the endometriosis model. TGF-β1, transforming growth factor-beta 1; α-SMA, alpha-smooth muscle actin; ELISA, enzyme-linked immunosorbent assay.
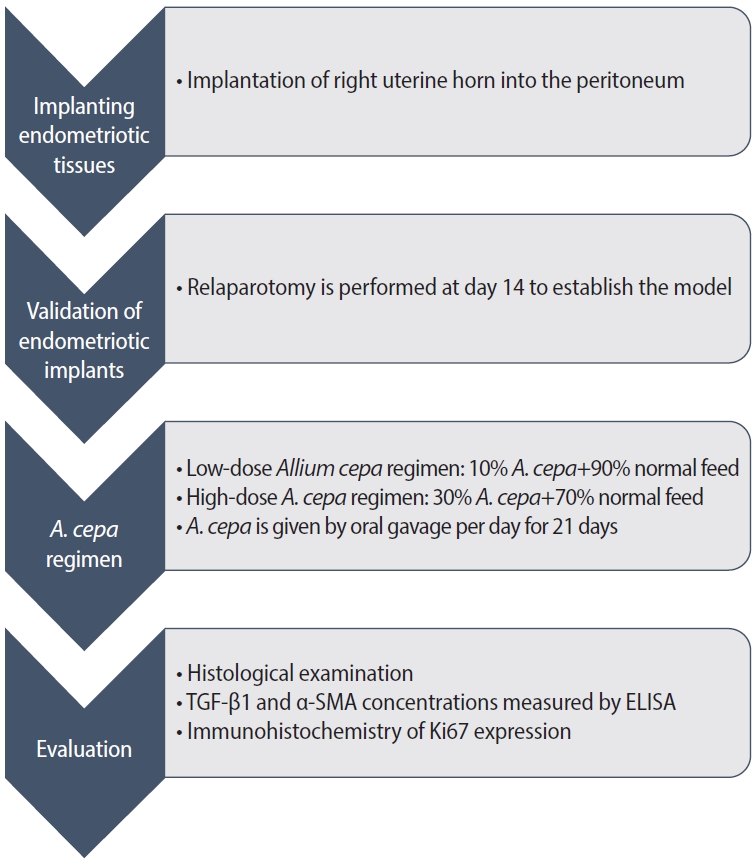
Figure 3.
Histopathologic images of the ovaries under hematoxylin and eosin staining (×10 magnification). (A) Endometriosis only. (B) Endometriosis+low-dose Allium cepa. (C) Endometriosis+high-dose A. cepa.
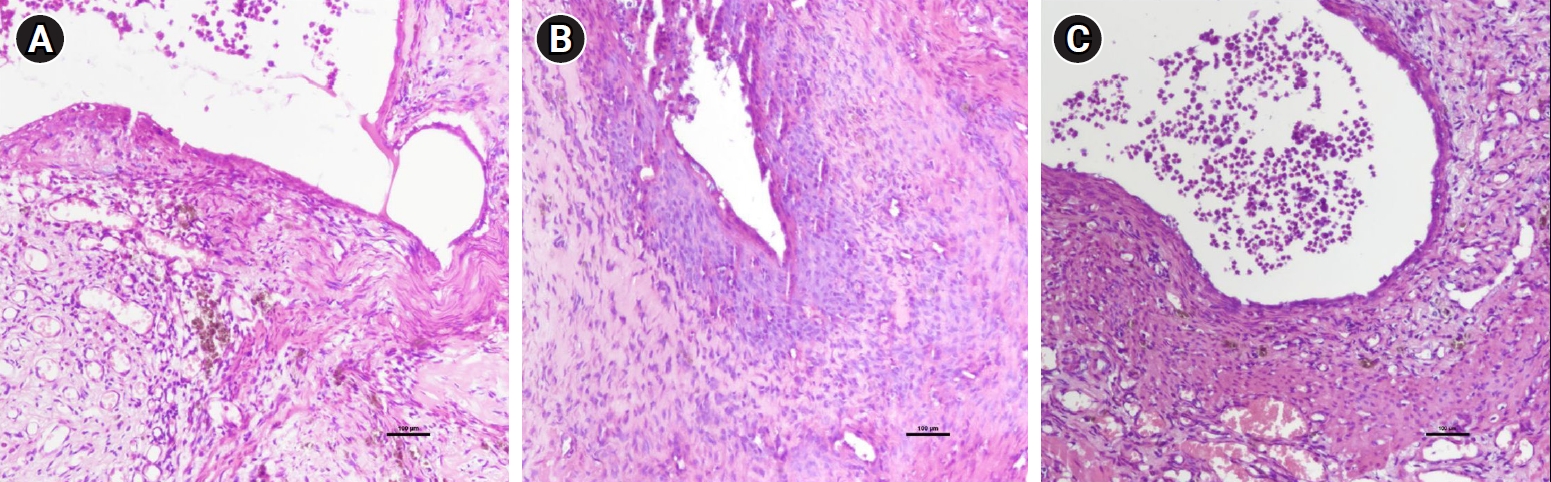
Figure 4.
Immunohistochemical analysis of Ki67 (×10 magnification). (A) Endometriosis only. (B) Endometriosis+low-dose Allium cepa. (C) Endometriosis+high-dose A. cepa.
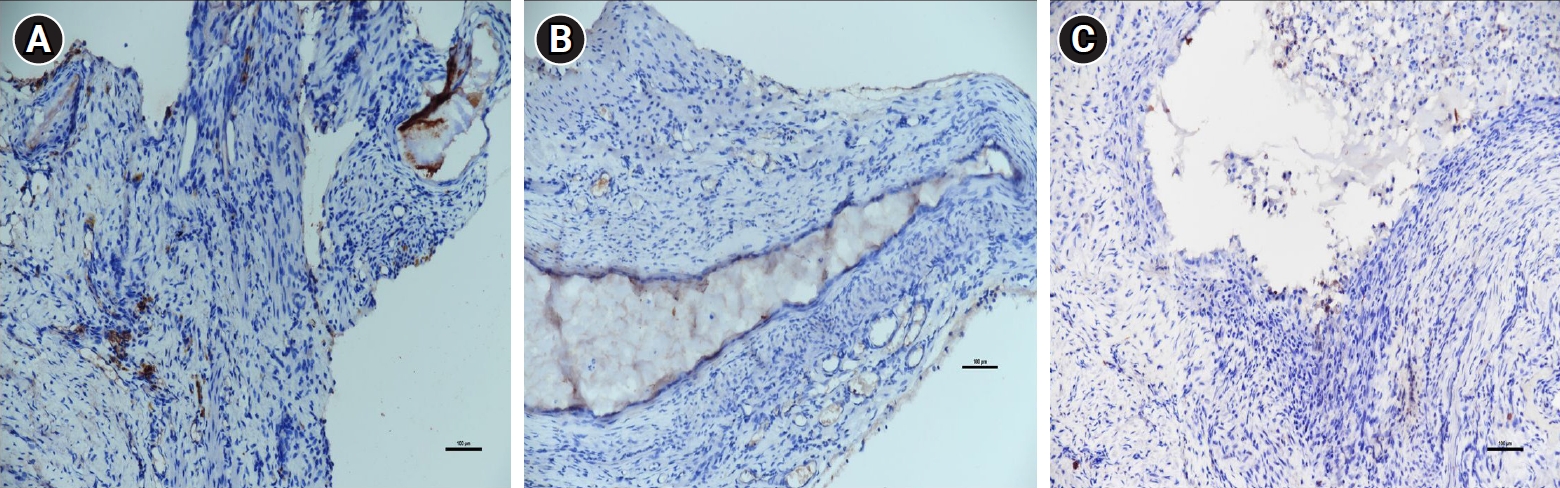
Table 1.
Mean histopathological evaluation scores
| Group | Histopathological evaluation score |
|---|---|
| Endometriosis only | 2.71±0.7 |
| Endometriosis+low-dose Allium cepa | 2.28±1.2 |
| Endometriosis+high-dose A. cepa | 1.14±1.0a) |
References
1. Taylor HS, Kotlyar AM, Flores VA. Endometriosis is a chronic systemic disease: clinical challenges and novel innovations. Lancet 2021;397:839-52.


3. Dun EC, Kho KA, Morozov VV, Kearney S, Zurawin JL, Nezhat CH. Endometriosis in adolescents. In: Nezhat CH, editor. Endometriosis in adolescents. Springer; 2020. p. 129-41.
4. Samimi M, Pourhanifeh MH, Mehdizadehkashi A, Eftekhar T, Asemi Z. The role of inflammation, oxidative stress, angiogenesis, and apoptosis in the pathophysiology of endometriosis: basic science and new insights based on gene expression. J Cell Physiol 2019;234:19384-92.



5. Burney RO. Fibrosis as a molecular hallmark of endometriosis pathophysiology. Fertil Steril 2022;118:203-4.


6. Malutan AM, Drugan T, Costin N, Ciortea R, Bucuri C, Rada MP, et al. Pro-inflammatory cytokines for evaluation of inflammatory status in endometriosis. Cent Eur J Immunol 2015;40:96-102.



7. Matsuzaki S, Darcha C, Maleysson E, Canis M, Mage G. Impaired down-regulation of E-cadherin and beta-catenin protein expression in endometrial epithelial cells in the mid-secretory endometrium of infertile patients with endometriosis. J Clin Endocrinol Metab 2010;95:3437-45.


8. Vigano P, Candiani M, Monno A, Giacomini E, Vercellini P, Somigliana E. Time to redefine endometriosis including its pro-fibrotic nature. Hum Reprod 2018;33:347-52.


9. Meligy FY, Elgamal DA, Abdelzaher LA, Khashbah MY, El-Mokhtar MA, Sayed AA, et al. Adipose tissue-derived mesenchymal stem cells reduce endometriosis cellular proliferation through their anti-inflammatory effects. Clin Exp Reprod Med 2021;48:322-36.




10. Yalcin SE, Ocal I, Yalcin Y, Selim HS, Caltekin MD, Aydogmus H, et al. Evaluation of the Ki-67 proliferation index and urocortin expression in women with ovarian endometriomas. Eurasian J Med 2017;49:107-12.



11. Galavi A, Hosseinzadeh H, Razavi BM. The effects of Allium cepa L. (onion) and its active constituents on metabolic syndrome: a review. Iran J Basic Med Sci 2021;24:3-16.


12. Lagana AS, Sofo V, Salmeri FM, Palmara VI, Triolo O, Terzic MM, et al. Oxidative stress during ovarian torsion in pediatric and adolescent patients: changing the perspective of the disease. Int J Fertil Steril 2016;9:416-23.

13. Marefati N, Ghorani V, Shakeri F, Boskabady M, Kianian F, Rezaee R, et al. A review of anti-inflammatory, antioxidant, and immunomodulatory effects of Allium cepa and its main constituents. Pharm Biol 2021;59:287-302.



14. Kula H, Ilgen O, Kurt S, Yilmaz F. Effects of Allium cepa on ovarian torsion-detorsion injury in a rat model. Turk J Obstet Gynecol 2023;20:137-41.



15. Park S, Lim W, Bazer FW, Whang KY, Song G. Quercetin inhibits proliferation of endometriosis regulating cyclin D1 and its target microRNAs in vitro and in vivo. J Nutr Biochem 2019;63:87-100.


16. Kocadal NC, Attar R, Yildirim G, Ficicioglu C, Ozkan F, Yilmaz B, et al. Melatonin treatment results in regression of endometriotic lesions in an ooferectomized rat endometriosis model. J Turk Ger Gynecol Assoc 2013;14:81-6.



17. Jouhari S, Mohammadzadeh A, Soltanghoraee H, Mohammadi Z, Khazali S, Mirzadegan E, et al. Effects of silymarin, cabergoline and letrozole on rat model of endometriosis. Taiwan J Obstet Gynecol 2018;57:830-5.


18. Keenan JA, Williams-Boyce PK, Massey PJ, Chen TT, Caudle MR, Bukovsky A. Regression of endometrial explants in a rat model of endometriosis treated with the immune modulators loxoribine and levamisole. Fertil Steril 1999;72:135-41.


19. Symons LK, Miller JE, Tyryshkin K, Monsanto SP, Marks RM, Lingegowda H, et al. Neutrophil recruitment and function in endometriosis patients and a syngeneic murine model. FASEB J 2020;34:1558-75.



20. Nasu K, Nishida M, Kawano Y, Tsuno A, Abe W, Yuge A, et al. Aberrant expression of apoptosis-related molecules in endometriosis: a possible mechanism underlying the pathogenesis of endometriosis. Reprod Sci 2011;18:206-18.



21. Hung SW, Zhang R, Tan Z, Chung JP, Zhang T, Wang CC. Pharmaceuticals targeting signaling pathways of endometriosis as potential new medical treatment: a review. Med Res Rev 2021;41:2489-564.




22. Onalan G, Tohma YA, Zeyneloglu HB. Effect of etanercept on the success of assisted reproductive technology in patients with endometrioma. Gynecol Obstet Invest 2018;83:358-64.



23. Cordaro M, Trovato Salinaro A, Siracusa R, D’Amico R, Impellizzeri D, Scuto M, et al. Hidrox and endometriosis: biochemical evaluation of oxidative stress and pain. Antioxidants (Basel) 2021;10:720.



24. Gordts S, Koninckx P, Brosens I. Pathogenesis of deep endometriosis. Fertil Steril 2017;108:872-85.


25. Ramalingam M, Kim H, Lee Y, Lee YI. Phytochemical and pharmacological role of liquiritigenin and isoliquiritigenin from Radix Glycyrrhizae in human health and disease models. Front Aging Neurosci 2018;10:348.



26. Hsu YW, Chen HY, Chiang YF, Chang LC, Lin PH, Hsia SM. The effects of isoliquiritigenin on endometriosis in vivo and in vitro study. Phytomedicine 2020;77:153214.


27. Dela Cruz C, Reis FM. The role of TGFβ superfamily members in the pathophysiology of endometriosis. Gynecol Endocrinol 2015;31:511-5.


28. Zhang Z, Suo L, Chen Y, Zhu L, Wan G, Han X. Endometriotic peritoneal fluid promotes myofibroblast differentiation of endometrial mesenchymal stem cells. Stem Cells Int 2019;2019:6183796.




29. Siracusa R, D’Amico R, Cordaro M, Peritore AF, Genovese T, Gugliandolo E, et al. The methyl ester of 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid reduces endometrial lesions development by modulating the NFkB and Nrf2 pathways. Int J Mol Sci 2021;22:3991.



30. Huang Q, Liu X, Guo SW. Higher fibrotic content of endometriotic lesions is associated with diminished prostaglandin E2 signaling. Reprod Med Biol 2021;21:e12423.




31. Oyewusi AJ, Oridupa OA, Saba AB, Oyewusi IK, Olukunle JO. Anti-inflammatory and analgesic effects of methanol extract of red cultivar Allium cepa bulbs in rats and mice. J Basic Clin Physiol Pharmacol 2021;32:1087-92.


32. Istrate-Ofiteru AM, Pirici D, Niculescu M, Berceanu C, Berceanu S, Voicu NL, et al. Clinical, morphological and immunohistochemical survey in different types of endometriosis. Rom J Morphol Embryol 2018;59:1133-53.