 |
 |
- Search
| Clin Exp Reprod Med > Volume 50(4); 2023 > Article |
|
Abstract
Objective
We evaluated the efficacy of the newly developed optimized in vitro culture (OIVC) dish for cultivating preimplantation mouse embryos. This dish minimizes the need for mineral oil and incorporates microwells, providing a stable culture environment and enabling independent monitoring of individual embryos.
Methods
Mouse pronuclear (PN) zygotes and two-cell-stage embryos were collected at 18 and 46 hours after human chorionic gonadotropin injection, respectively. These were cultured for 120 hours using potassium simplex optimized medium (KSOM) to reach the blastocyst stage. The embryos were randomly allocated into three groups, each cultured in one of three dishes: a 60-mm culture dish, a microdrop dish, and an OIVC dish that we developed.
Results
The OIVC dish effectively maintained the osmolarity of the KSOM culture medium over a 5-day period using only 2 mL of mineral oil. This contrasts with the significant osmolarity increase observed in the 60-mm culture dish. Additionally, the OIVC dish exhibited higher blastulation rates from two-cell embryos (100%) relative to the other dish types. Moreover, blastocysts derived from both PN zygotes and two-cell embryos in the OIVC dish group demonstrated significantly elevated mean cell numbers.
Conclusion
Use of the OIVC dish markedly increased the number of cells in blastocysts derived from the in vitro culture of preimplantation mouse embryos. The capacity of this dish to maintain medium osmolarity with minimal mineral oil usage represents a breakthrough that may advance embryo culture techniques for various mammals, including human in vitro fertilization and embryo transfer programs.
Human assisted reproductive technology (ART) has progressed to include advanced methods, including in vitro fertilization (IVF) and in vitro culture techniques for mammalian embryos. in vitro embryo development requires the consideration of numerous factors that differ substantially from those in vivo [1]. The success of these cultures, particularly when applied to sensitive preimplantation embryos, largely depends on the stability of the culture environment. This includes factors such as osmolarity, temperature, and gas concentrations [1]. Traditional oil-drop culture methods employ mineral oil overlays to prevent the evaporation and maintain the osmolarity of the culture medium [2]. However, the excessive use of mineral oil can pose challenges, including high costs, variability in oil quality, and potential contamination [2].
For decades, communal culture of multiple embryos was a widely used method in in vitro embryo culture due to its numerous advantages [3,4]. In essence, cultured embryos thrive when grouped together. The communal culture method has been shown to positively impact blastocyst rates and subsequent in vivo development. The primary mechanisms believed to enhance development in group culture include accommodation, communication, and protection [5-7]. Accommodation refers to the capacity of embryos to adapt to their surroundings. In communal culture, embryos encounter a more varied environment, potentially fostering greater resilience [8]. Communication involves the exchange of signals between embryos, which may aid in synchronizing their development and ensuring their progression at a similar pace [9,10]. Finally, protection indicates that embryos in communal culture are less susceptible to damage from environmental stressors than are individual embryos. This is attributed to the surrounding embryos, which help serve as a shield against harmful factors [11-18].
However, the current approach to embryo culture has evolved, with individual culture now being the preferred method among laboratories [18]. This shift is largely because most methods of identifying embryos with the highest developmental competence necessitate individual follow-up screenings [19]. Furthermore, with the rising average patient age and the reduced intensity of stimulation in mild protocols, the number of zygotes may be decreased to the point that group effects are not present. This suggests that the advantages of group culture, such as nutrient and growth factor sharing, may not be as pronounced for older patients or those undergoing mild stimulation [20]. The presence of degenerated or dead embryos can also impede the development of healthy ones, as these can release harmful chemicals into the culture medium, potentially damaging the developing embryos [21].
Oil droplet culture is a widely used method for culturing individual preimplantation embryos in both mice and humans. This technique can help minimize potentially harmful changes in osmotic pressure and pH by preventing the evaporation of the medium. Furthermore, the oil layer can inhibit the dispersion of essential nutrients and growth factors away from the embryo. However, the specifics of this method are not yet fully understood. For instance, the required volume of medium is not well established and may differ based on the laboratory and culture conditions [22]. Additionally, the high surface-to-volume ratio of the droplets could potentially result in nutrient loss and the accumulation of toxic waste.
The well-of-the-well (WOW) system is a novel embryo culture technology designed to enhance embryo health and improve pregnancy rates by facilitating individual embryo culture. Originally developed for bovine embryo culture [23], the WOW system has since been adapted for use with other animal embryos. It has been demonstrated to promote embryo growth, improve quality, and minimize damage [24]. Furthermore, the WOW system has been found to augment both embryo fertilization rates and pregnancy rates in human ART programs [25].
The objective of this study was to assess the effectiveness of the newly developed optimized in vitro culture (OIVC) dish for cultivating preimplantation mouse embryos. The performance of the OIVC dish was assessed based on its capacity to maintain osmolarity and developmental outcomes in mouse pronuclear (PN) zygotes and two-cell-stage embryos. The results were compared to other commercially available culture dishes that employ the conventional oil-drop culture method.
This study received approval from the Eulji University Institutional Animal Care and Use Committee (No. EUIACUC 21-23). The protocol for superovulating mice was outlined by Park et al. [26]. In brief, female BDF (mouse strain) or ICR (mouse strain) mice aged between 6 and 9 weeks were superovulated using intraperitoneal injections of 5 IU of pregnant mareŌĆÖs serum gonadotropin (Prospec). After 48 hours, these mice received an additional injection of 5 IU of human chorionic gonadotropin (hCG; Prospec). Then, each superovulated mouse was individually mated with a fertile male BDF or ICR mouse.
Approximately 18 to 19 hours after hCG injection and mating, BDF female mice with a confirmed vaginal plug were euthanized via cervical dislocation. Subsequently, cumulus-enclosed one-cell embryos (PN zygotes) were collected from the oviductal ampullae. These zygotes were then denuded via 1 minute of incubation with 0.1% hyaluronidase (Sigma-Aldrich) in phosphate-buffered saline (PBS; Welgene). Following this, the zygotes were pooled and washed three times in potassium simplex optimized medium (KSOM, MR-121; Sigma-Aldrich) supplemented with 0.4% bovine serum albumin (BSA; Sigma-Aldrich).
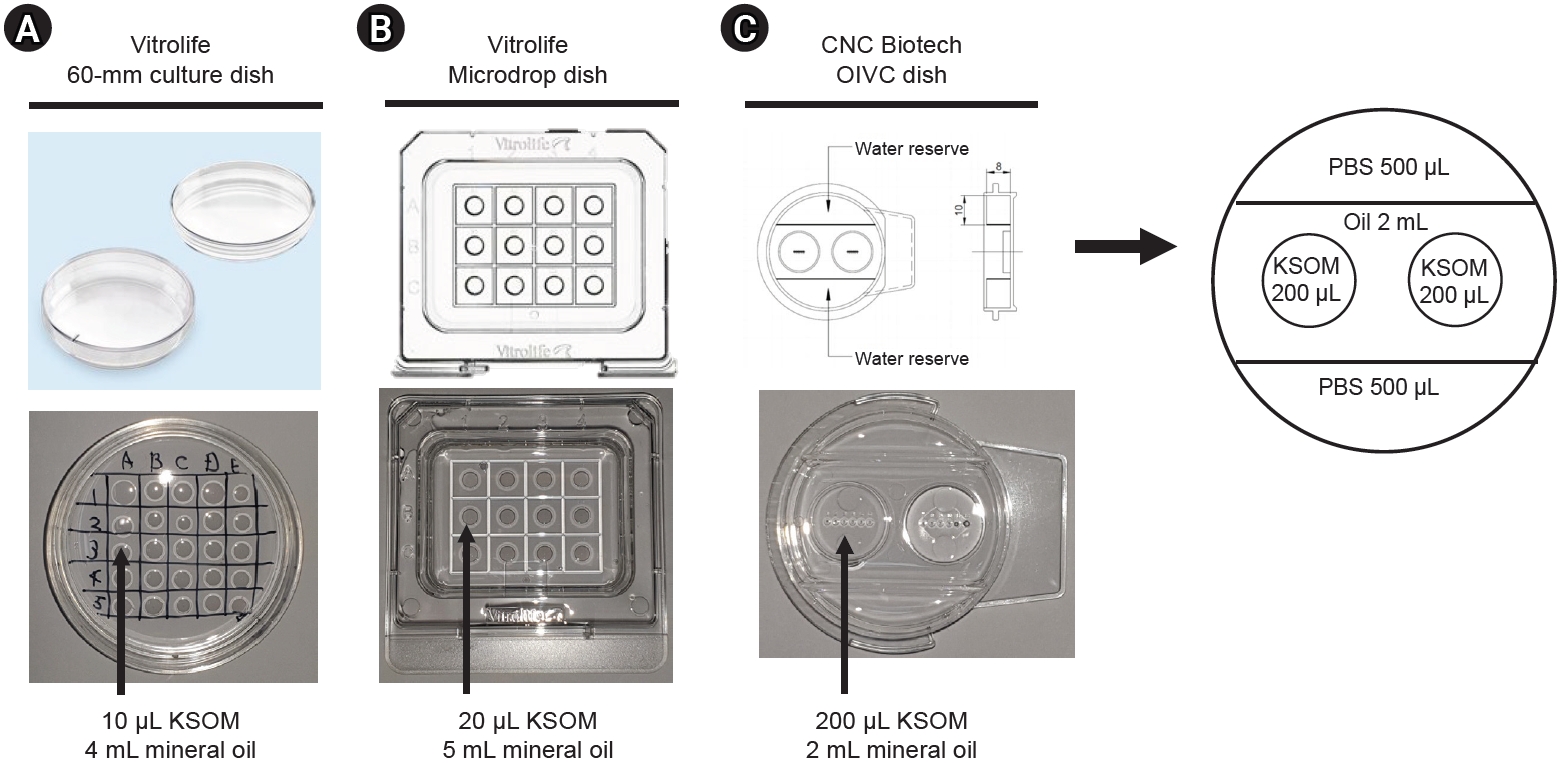
Two-cell-stage embryos were obtained from ICR female mice through oviduct flushing, approximately 42 to 44 hours after hCG injection. Healthy zygotes and two-cell-stage embryos were individually cultured in a droplet of KSOM medium containing 0.4% BSA, which was covered with light mineral oil (9305; FujiFilm Irvine Scientific). This was done using three distinct types of culture dishes. The embryos were randomly assigned and cultured in three groups, each corresponding to a different dish type as illustrated in Figure 1: (1) a 60-mm culture dish (16002; Vitrolife) with 10-╬╝L droplets of KSOM overlaying 4 mL of mineral oil; (2) a microdrop dish (16003; Vitrolife) with 20-╬╝L droplets of KSOM overlaying 5 mL of mineral oil; and (3) an OIVC dish (CNC Biotech) featuring six small wells, each containing 200 ╬╝L of KSOM overlaying 2 mL of mineral oil. The mouse embryos were cultured in vitro in a forma direct heat CO2 Incubator (Thermo Fisher Scientific), a device with regulatory approval, under standard embryonic culture conditions at 37 ┬░C with 5% CO2.
The osmolarity of the media was measured using the freezing-point depression method with an automatic osmometer (Micro Osmometer 3300; Advanced Instruments), in accordance with the manufacturerŌĆÖs instructions. After 5 days of in vitro culture, the culture media from each of the three types of dishes were collected and measured at least three times each.
The cleavage rate from the PN zygote to the two-cell stage was determined 24 hours after PN zygote collection. The blastulation rates of PN zygotes and two-cell-stage embryos were ascertained 96 and 72 hours after embryo collection, respectively. Following observation, the blastocysts were fixed with 4% paraformaldehyde. The nuclei of the blastocysts were then stained and counted using a solution of 10 mg/mL bisbenzimide (Hoechst 33342; Sigma-Aldrich) under a fluorescence microscope (AX-70; Olympus).
All experiments were performed at least in triplicate. Depending on the data format, group comparisons were made using either the chi-square test or one-way analysis of variance. The Tukey honestly significant difference post hoc test was employed for all group comparisons. A p-value of less than 0.05 was considered to indicate statistical significance.
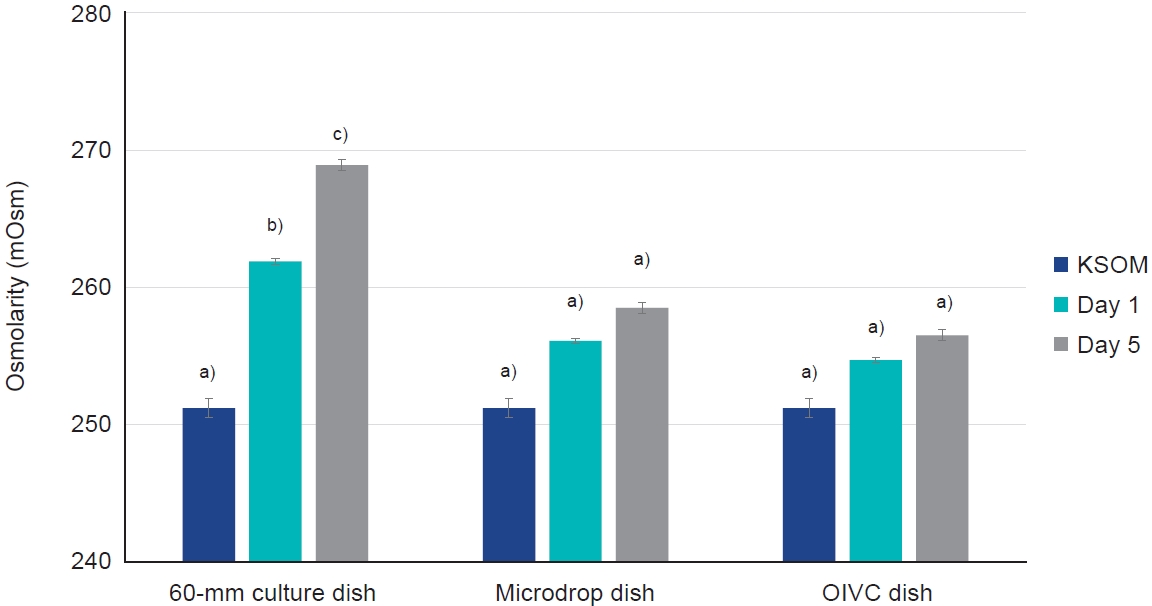
The osmolarity of the KSOM culture media in the OIVC dish, covered with only 2 mL of mineral oil, was effectively maintained over a 5-day in vitro culture period. This contrasted with the 60-mm culture dish, which displayed a significant increase in osmolarity (p<0.05) (Figure 2). More specifically, the osmolarity in the OIVC dish was consistently maintained over the 5 days of in vitro culture, ranging from 251.2┬▒0.7 to 256.5┬▒0.4 mOsm. However, the osmolarity of the KSOM culture media in the 60-mm culture dish significantly increased from 251.2┬▒0.7 to 258.9┬▒0.4 mOsm over the same period (p<0.05) (Table 1).
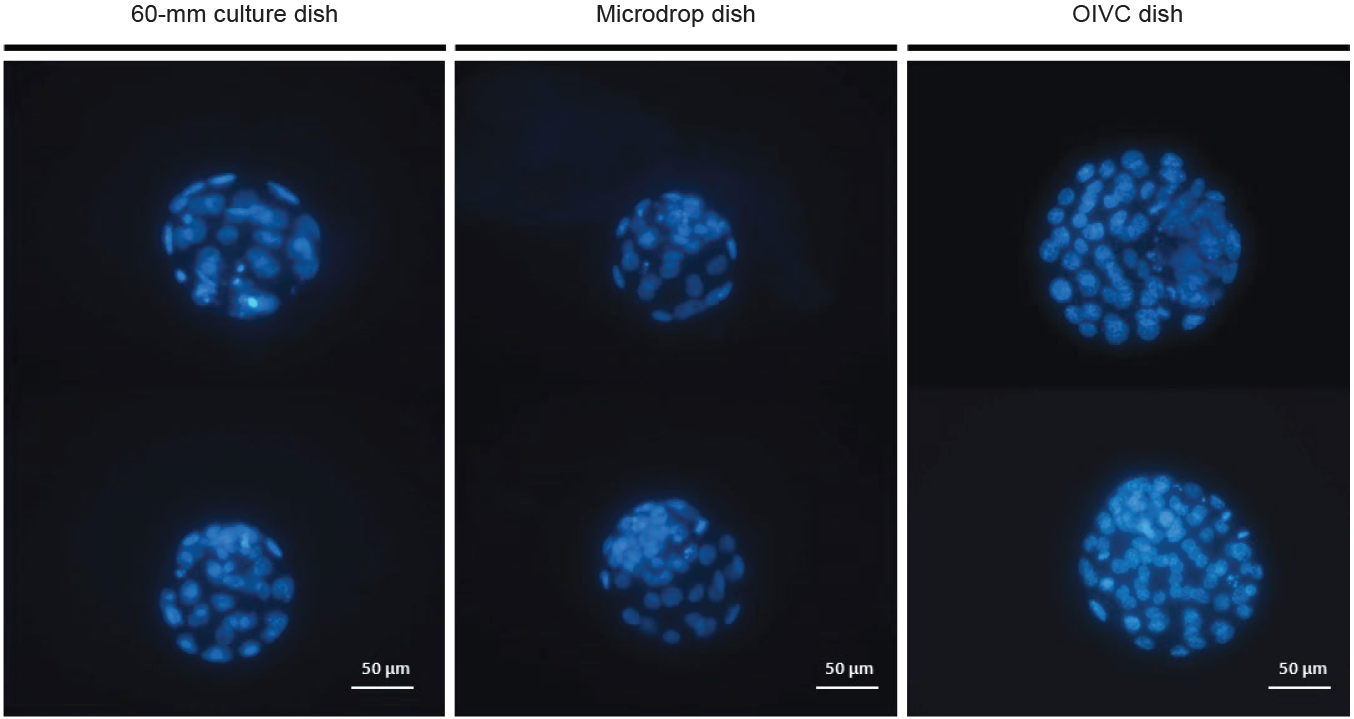
The rates of mouse embryonic development were evaluated at intervals of 24 hours (corresponding to development to the two-cell stage) and 96 hours (corresponding to development to the blastocyst stage). These results are displayed in Table 2, Figure 3. The cleavage rate was significantly higher in the 60-mm culture dish compared to the microdrop dish (p<0.05), but it was comparable between the 60-mm dish and the OIVC dish. Similar patterns were observed in the blastulation rates across groups. However, the total cell counts of blastocysts were significantly higher in the OIVC dish (70.6┬▒1.9) relative to both the 60-mm culture dish (46.1┬▒2.0) and the microdrop dish (47.4┬▒1.9; p<0.05) (Table 2). The cells of the blastocysts were stained with bisbenzimide and examined under a fluorescent microscope, as shown in Figure 4.
The rates of mouse embryonic development were evaluated after culturing from the two-cell stage to the blastocyst stage, and these results are displayed in Table 3. The OIVC dish demonstrated high blastulation rates, although no significant differences were observed among the groups. The blastulation rates were 100% for the OIVC dish, 95.7% for the microdrop dish, and 95.8% for the 60-mm culture dish. However, the total cell counts of the blastocysts were significantly higher in the OIVC dish (84.5┬▒2.0) compared to the 60-mm culture dish (71.1┬▒2.1) and the microdrop dish (77.3┬▒2.6; p<0.05) (Table 3).
Numerous embryologists have pursued research aimed at enhancing the success rate of human embryonic development and ART. In a series of prior studies, the impact of fertilization methods on morphological events and abnormal division during embryonic development was examined. The findings indicated differences up to the six-cell stages between embryos derived from IVF and those obtained from intracytoplasmic sperm injection [27]. Another study delved into embryonic development, morphology, and clinical outcomes, revealing higher success rates in regularly developed blastocysts as opposed to those with morphokinetic variables [28]. In a study investigating the effects of oxygen, a novel gas-supplied incubator was utilized, and dynamic oxygen concentrations were observed to improve mouse embryonic development [29]. Additionally, we conducted a review on non-invasive embryonic quality evaluation using time-lapse imaging, artificial intelligence, molecular markers, and nucleic acid analysis. The review underscored the necessity for robust clinical trials to integrate and validate various methods for comprehensive evaluation [30]. In the present study, we introduced a newly designed culture dish for the in vitro culture of preimplantation mammalian embryos, aimed at increasing the success rate of embryonic development.
The OIVC dish is a novel design aimed at reducing the use of mineral oil and maintaining the osmolarity of the culture medium more effectively than other dishes used for in vitro preimplantation embryo culture. In fact, the OIVC dish demonstrated a 50% reduction in mineral oil usage compared to a standard 60-mm culture dish and a 60% reduction compared to a microdrop dish. This study revealed that the use of the OIVC dish yielded substantial improvements in blastulation rates and the number of cells in blastocysts from in vitro culture of mouse PN and two-cell-stage embryos.
Mineral oil plays a crucial role in the in vitro culture of preimplantation embryos, providing protection against osmotic stress by preventing medium evaporation [25]. However, the drawbacks of using mineral oil are well-documented. Variations in quality and the presence of potential pollutants can lead to inconsistent results and an increased risk of infection [20,31]. Osmolarity, a measure of the concentration of dissolved particles in a solution, is a critical factor in cell proliferation and embryo development in vitro. If the osmolarity of the culture medium becomes too high or too low, the embryos may be harmed. Numerous studies have underscored the importance of maintaining the osmolarity in embryo culture media, with research conducted on mouse, porcine, bovine, and human models.
Prior research has also highlighted the role of osmolarity in various contexts. For instance, when preserving rabbit embryos, it was found that maintaining osmolarity within a specific range (285 to 340 mOsm) was crucial for effective preservation. Any deviation from this range resulted in structural changes to the embryos [32]. In the context of embryo culture, adjustments to osmolarity can aid in overcoming the two-cell block [33]. This can be achieved by fine-tuning the components of the culture medium, such as sodium chloride (NaCl), potassium chloride, and glucose, indicating that osmolarity is not the sole factor influencing embryo development [34]. Similarly, the development of mouse zygotes is also influenced by osmolarity. Elevated NaCl concentrations can impede development, whereas the presence of glutamine/betaine can offer protection against this effect [35]. The development of bovine embryos is likewise linked to NaCl concentration, with specific ranges promoting development. Adjustments to osmolarity can influence different stages of development, highlighting its importance [36]. In the case of porcine nuclear transfer and IVF embryos, early manipulation of osmolarity can impact development and gene expression, potentially enhancing growth and reducing apoptosis [37]. In summary, osmolarity has emerged as a key factor in these studies, influencing various aspects of embryo development, preservation, and gene expression.
Maintaining osmotic balance is crucial for optimal embryonic development [38]. Substantial changes in the osmotic pressure of the culture, which is designed to be suitable for embryo development during in vitro culture, can induce physiological stress and impact the morphology, viability, and implantation potential of the embryo [39]. The OIVC plate offers an advantage in this regard, as it can consistently maintain osmotic pressure while minimizing the use of mineral oil, making it highly durable. By inhibiting excessive evaporation of the culture, it ensures the maintenance of appropriate osmotic pressure, thereby creating a stable environment that mitigates stress risk and improves the prospects of embryonic survival and implantation.
From a pragmatic perspective, decreasing the usage of mineral oil results in cost savings, particularly in high-volume IVF clinics where mineral oil is a costly reagent. Furthermore, minimizing the handling of mineral oil can enhance workflow efficiency, streamlining the processes within the IVF clinic.
The integration of time-lapse systems into the in vitro culture of preimplantation embryos represents a key advancement, enabling continuous monitoring of embryonic development without the need for manual evaluation [40]. The potential to combine this time-lapse technology with the OIVC dish could offer several benefits. First, continuous non-invasive monitoring using the OIVC dish could facilitate uninterrupted observation of individual embryos, providing valuable data on crucial developmental milestones such as the timing of cell division, which is indicative of embryonic viability [41]. Second, time-lapse monitoring can enhance the accuracy of embryo selection by identifying subtle morphological changes and anomalies that may be missed during fixed-time evaluations [42]. Third, an optimized culture environment, achieved by maintaining osmotic pressure and minimizing the use of mineral oil, ensures that time-lapse records are captured under consistent conditions that are conducive to optimal embryonic development during in vitro culture. These synergistic benefits not only bridge the gap between research and clinical embryology, but also have the potential to revolutionize our understanding of early mammalian development in vitro. While the present findings are focused on mouse embryos, they could considerably improve embryonic culture practices by balancing cost-effectiveness with optimal conditions.
Adding to its value, the OIVC dish incorporates innovative embryonic culture techniques. These methods improve embryonic health and pregnancy rates, while also being cost-effective and environmentally stable. Furthermore, they facilitate research into early mammalian development through integration with time-lapse systems. The dish provides a stable microwell-based culture environment, minimizing the use of mineral oils. This commitment to media stability, individualized culture, and embryo tracking begins with individualized cultural concepts such as WOW systems. The effectiveness of OIVC plates is demonstrated through the evaluation of osmotic retention and developmental outcomes in mouse PN junctions and two-cell-stage embryos, showing equivalent effectiveness to standard methods [33]. Additionally, the capacity to culture individual embryos offers the potential for optimized embryonic selection for quality evaluation and metastasis.
The OIVC dish, a new innovation within the WOW system, was designed for the in vitro culture of mammalian preimplantation embryos, including those from mice and humans. This cutting-edge dish reduces the reliance on mineral oil and includes microwells, which provide a stable culture medium environment and allow for the individual monitoring of each embryo. Moreover, it enables differential cultivation between groups by partitioning the microwells into two sections, facilitating a direct comparison of the conditions within each group. The dish also incorporates double microwells, which allow for both individual and group cultivation by linking the groups to the culture medium. The OIVC dish was designed to effectively prevent oil and media evaporation during in vitro culture. This is achieved by placing supplemented culture medium or other non-toxic solutions, such as PBS or distilled water, on the sides of the dish to maintain osmotic pressure. This innovative design concept was applied to the development of a new OIVC plate.
In conclusion, our research demonstrates that the OIVC dish yielded significant improvements in blastulation rates and the number of cells in blastocysts during the in vitro culture of mouse PN and two-cell-stage embryos. Its capacity to maintain media osmolarity while reducing the use of mineral oil represents a breakthrough with the potential to enhance embryo culture techniques, including those relevant to human IVF and embryo transfer programs. Further research is necessary to confirm these results, evaluate long-term safety and effectiveness, and investigate the potential for a revolutionary change in the embryonic culture system.
Notes
Figure┬Ā1.
Dishes utilized in this study. (A) A 60-mm culture dish (Vitrolife, 16002) containing 10-╬╝L drops of potassium simplex optimized medium (KSOM), overlaid with 4 mL of mineral oil. (B) A microdrop dish (Vitrolife, 16003) with 20-╬╝L drops of KSOM, overlaid with 5 mL of mineral oil. (C) An optimized in vitro culture (OIVC) dish (CNC Biotech) featuring six small wells, each containing 200 ╬╝L of KSOM overlaid with 2 mL of mineral oil. PBS, phosphate-buffered saline.
Figure┬Ā2.
Osmolarity of potassium simplex optimized medium (KSOM) culture media in three types of dishes following 5 days of in vitro culture. OIVC, optimized in vitro culture. One-way analysis of variance was used, along with the Tukey multiple comparison test, to compare groups a), b), and c) (p<0.05).
Figure┬Ā3.
Mouse embryonic development rates after culture were assessed at intervals of 24 hours, which corresponds to development to the two-cell stage, and 96 hours, which corresponds to development to the blastocyst stage. OIVC, optimized in vitro culture; hCG, human chorionic gonadotropin.
Figure┬Ā4.
Total blastocyst cell counts were significantly higher in the OIVC dish (70.6┬▒1.9) compared to both the 60-mm culture dish (46.1┬▒2.0) and the microdrop dish (47.4┬▒1.9) (├Ś400 magnification). OIVC, optimized in vitro culture.
Table┬Ā1.
Osmolarity of KSOM culture media in three types of dishes following 5 days of in vitro culture
Table┬Ā2.
Assessment of mouse embryonic development rates from zygotes following culture every 24 hours (corresponding to development to the two-cell stage) and 96 hours (corresponding to development to the blastocyst stage)
Table┬Ā3.
Assessment of mouse embryonic development rates following culture from the two-cell stage to the blastocyst stage
References
1. Wale PL, Gardner DK. The effects of chemical and physical factors on mammalian embryo culture and their importance for the practice of assisted human reproduction. Hum Reprod Update 2016;22:2-22.


2. Scarica C, Monaco A, Borini A, Pontemezzo E, Bonanni V, De Santis L, et al. Use of mineral oil in IVF culture systems: physico-chemical aspects, management, and safety. J Assist Reprod Genet 2022;39:883-92.




3. Wiley LM, Yamami S, Van Muyden D. Effect of potassium concentration, type of protein supplement, and embryo density on mouse preimplantation development in vitro. Fertil Steril 1986;45:111-9.


4. Ruiz M, Santamaria-Lopez E, Blasco V, Hernaez MJ, Caligara C, Pellicer A, et al. Effect of group embryo culture under low-oxygen tension in benchtop incubators on human embryo culture: prospective, randomized, controlled trial. Reprod Sci 2020;27:1522-33.



5. Stojanov T, Alechna S, O'Neill C. In-vitro fertilization and culture of mouse embryos in vitro significantly retards the onset of insulin-like growth factor-II expression from the zygotic genome. Mol Hum Reprod 1999;5:116-24.


6. Gopichandran N, Leese HJ. The effect of paracrine/autocrine interactions on the in vitro culture of bovine preimplantation embryos. Reproduction 2006;131:269-77.


7. Salahuddin S, Ookutsu S, Goto K, Nakanishi Y, Nagata Y. Effects of embryo density and co-culture of unfertilized oocytes on embryonic development of in-vitro fertilized mouse embryos. Hum Reprod 1995;10:2382-5.

8. Johnson MH, Nasr-Esfahani MH. Radical solutions and cultural problems: could free oxygen radicals be responsible for the impaired development of preimplantation mammalian embryos in vitro? Bioessays 1994;16:31-8.


9. Joo BS, Kim MK, Na YJ, Moon HS, Lee KS, Kim HD. The mechanism of action of coculture on embryo development in the mouse model: direct embryo-to-cell contact and the removal of deleterious components. Fertil Steril 2001;75:193-9.


10. Paria BC, Dey SK. Preimplantation embryo development in vitro: cooperative interactions among embryos and role of growth factors. Proc Natl Acad Sci U S A 1990;87:4756-60.



11. Kane MT, Carney EW, Ellington JE. The role of nutrients, peptide growth factors and co-culture cells in development of preimplantation embryos in vitro. Theriogenology 1992;38:297-313.


12. Gandolfi F. Autocrine, paracrine and environmental factors influencing embryonic development from zygote to blastocyst. Theriogenology 1994;41:95-100.

13. Christianson MS, Zhao Y, Shoham G, Granot I, Safran A, Khafagy A, et al. Embryo catheter loading and embryo culture techniques: results of a worldwide Web-based survey. J Assist Reprod Genet 2014;31:1029-36.




14. Fancsovits P, Pribenszky C, Lehner A, Murber A, Kaszas Z, Nemes A, et al. Prospective-randomized study comparing clinical outcomes of IVF treatments where embryos were cultured individually or in a microwell group culture dish. Biol Futur 2022;73:229-36.



15. Reed ML, Woodward BJ, Swain JE. Single or group culture of mammalian embryos: the verdict of the literature. J Reprod Biotechnol Fertil 2011;2:77-87.


16. Herreros M, Marti L, Diaz N, Tio MC, Rodriguez-Arnedo A, Guerrero J, et al. Impact of group embryo culture vs individual embryo culture strategies on blastocyst rate and quality. Hum Reprod 2022;37(Supplement 1): deac104.077.

17. De Munck N, Santos-Ribeiro S, Mateizel I, Verheyen G. Reduced blastocyst formation in reduced culture volume. J Assist Reprod Genet 2015;32:1365-70.




18. Minasi MG, Fabozzi G, Casciani V, Lobascio AM, Colasante A, Scarselli F, et al. Improved blastocyst formation with reduced culture volume: comparison of three different culture conditions on 1128 sibling human zygotes. J Assist Reprod Genet 2015;32:215-20.




19. Tagawa M, Matoba S, Narita M, Saito N, Nagai T, Imai K. Production of monozygotic twin calves using the blastomere separation technique and Well of the Well culture system. Theriogenology 2008;69:574-82.


20. Vajta G, Peura TT, Holm P, Paldi A, Greve T, Trounson AO, et al. New method for culture of zona-included or zona-free embryos: the Well of the Well (WOW) system. Mol Reprod Dev 2000;55:256-64.


21. Swain JE, Pool TB. ART failure: oocyte contributions to unsuccessful fertilization. Hum Reprod Update 2008;14:431-46.


22. Biggers JD, Summers MC. Choosing a culture medium: making informed choices. Fertil Steril 2008;90:473-83.


23. Wood SA, Allen ND, Rossant J, Auerbach A, Nagy A. Non-injection methods for the production of embryonic stem cell-embryo chimaeras. Nature 1993;365:87-9.



25. Ieda S, Akai T, Sakaguchi Y, Shimamura S, Sugawara A, Kaneda M, et al. A microwell culture system that allows group culture and is compatible with human single media. J Assist Reprod Genet 2018;35:1869-80.




26. Park JC, Kim JA, Kim DJ, Bae JG, Kim JI, Rhee JH. Effect of human hydrosalpingeal fluid on the development of mouse embryo. Korean J Reprod Med 2010;37:125-34.
27. Kim HJ, Yoon HJ, Jang JM, Lee WD, Yoon SH, Lim JH. Evaluation of human embryo development in in vitro fertilization- and intracytoplasmic sperm injection-fertilized oocytes: a time-lapse study. Clin Exp Reprod Med 2017;44:90-5.




28. Hur YS, Ryu EK, Hyun CS, Yang SH, Yoon SH, Lim KS, et al. Retrospective study of single vitrified-warmed blastocyst transfer cycles according to the presence of morphokinetic variables. Clin Exp Reprod Med 2018;45:52-5.




29. Lee SC, Seo HC, Lee J, Jun JH, Choi KW. Effects of dynamic oxygen concentrations on the development of mouse pre- and peri-implantation embryos using a double-channel gas supply incubator system. Clin Exp Reprod Med 2019;46:189-96.




30. Kim J, Lee J, Jun JH. Non-invasive evaluation of embryo quality for the selection of transferable embryos in human in vitro fertilization-embryo transfer. Clin Exp Reprod Med 2022;49:225-38.




31. Morbeck DE, Krisher RL, Herrick JR, Baumann NA, Matern D, Moyer T. Composition of commercial media used for human embryo culture. Fertil Steril 2014;102:759-66.


32. Hegele-Hartung C, Piegsa K, Fischer B. Effect of osmolarity of the fixative on the ultrastructure of preimplantation rabbit embryos. Acta Anat (Basel) 1989;136:79-88.



33. Lawitts JA, Biggers JD. Overcoming the 2-cell block by modifying standard components in a mouse embryo culture medium. Biol Reprod 1991;45:245-51.


34. Biggers JD, Lawitts JA, Lechene CP. The protective action of betaine on the deleterious effects of NaCl on preimplantation mouse embryos in vitro. Mol Reprod Dev 1993;34:380-90.


35. Lim JM, Kim JH, Okuda K, Niwa K. The importance of NaCl concentration in a chemically defined medium for the development of bovine oocytes matured and fertilized in vitro. Theriogenology 1994;42:421-32.


36. Hwang IS, Park MR, Moon HJ, Shim JH, Kim DH, Yang BC, et al. Osmolarity at early culture stage affects development and expression of apoptosis related genes (Bax-alpha and Bcl-xl) in pre-implantation porcine NT embryos. Mol Reprod Dev 2008;75:464-71.


37. Lane M, Gardner DK. Understanding cellular disruptions during early embryo development that perturb viability and fetal development. Reprod Fertil Dev 2005;17:371-8.


38. Valojerdi MR, Karimian L, Yazdi PE, Gilani MA, Madani T, Baghestani AR. Efficacy of a human embryo transfer medium: a prospective, randomized clinical trial study. J Assist Reprod Genet 2006;23:207-12.




39. Meseguer M, Herrero J, Tejera A, Hilligsoe KM, Ramsing NB, Remohi J. The use of morphokinetics as a predictor of embryo implantation. Hum Reprod 2011;26:2658-71.


40. Rubio I, Kuhlmann R, Agerholm I, Kirk J, Herrero J, Escriba MJ, et al. Limited implantation success of direct-cleaved human zygotes: a time-lapse study. Fertil Steril 2012;98:1458-63.









