The effects of berberine on ischemia-reperfusion injuries in an experimental model of ovarian torsion
Article information
Abstract
Objective
Ovarian torsion is a gynecological disorder that causes ischemia-reperfusion injuries in the ovary. Our study investigated berberine's short- and long-term effects on ovarian ischemia-reperfusion injuries.
Methods
This study included 28 Wistar albino female rats weighing 180 to 220 g, which were divided into four groups: sham (S), torsion/detorsion (T/D), torsion/detorsion+single dose berberine (T/D+Bb), and torsion/detorsion+15 days berberine (T/D+15Bb). The torsion and detorsion model was applied in all non-sham groups. In the T/D+Bb group, a single dose of berberine was administered, while in the T/D+15Bb group, berberine was administered over a period of 15 days. After the rats were euthanized, their ovaries were excised. The left ovaries were used for histopathologic evaluation, which included ovarian injury scoring and follicle count, while the right ovaries were used for biochemical analyses (tissue transforming growth factor-β [TGF-β] and alpha-smooth muscle actin [α-SMA] levels).
Results
The histopathologic evaluation scores for the ovaries were significantly lower in the T/D+B group (p<0.05) and the T/D+15B group (p<0.005) than in the T/D group. The follicle counts in the T/D group were lower than those in both the sham and treated groups (p<0.005). The TGF-β levels were significantly lower in the T/D+15B group (p<0.005), whereas the α-SMA levels did not show a significant difference.
Conclusion
Both short- and long-term berberine use could potentially have therapeutic effects on ovarian torsion. Long-term berberine use exhibited anti-inflammatory effects by reducing TGF-β levels, thereby preventing ischemia-reperfusion injuries. Therefore, we suggest that long-term berberine use could be beneficial for ovarian torsion.
Introduction
Adnexal torsion is defined as rotation of the ovary and fallopian tube around their own axis. This condition is primarily observed during the reproductive period and is considered a gynecological emergency due to its potential for serious complications. The adnexa, composed of the fallopian tubes and ovaries, may experience torsion either together or independently [1]. The etiology of the ischemic injury that can result from torsion is believed to be oxidative stress and inflammation caused by reactive oxygen metabolites in the surrounding environment. This phase, known as the ischemic injury period, is primarily associated with ovarian injury due to hypoxia. Once the torsion is alleviated, the reperfusion injury process commences, characterized by the production of reactive oxygen and nitrogen compounds. The total tissue damage is thought to be the cumulative result of injuries caused by both ischemia and reperfusion. Therefore, successful prevention of reperfusion injury can significantly enhance the effectiveness of ischemia treatment [2].
Free oxygen radicals are produced during ischemia and reperfusion, leading to oxidative stress [2]. It is believed that ischemia and free oxygen radicals, resulting from torsion, are the primary causes of diminished ovarian reserve. These injuries can potentially lead to follicular cell degeneration in the ovary, vascular congestion, hemorrhage, inflammation, and tissue loss, as evidenced by histopathological findings [3]. From this perspective, antioxidant agents have been explored in the literature as potential adjuncts to surgical treatment for torsion [4-8].
Berberine is a non-basic, herbal quaternary benzylisoquinoline alkaloid with a well-documented history in Ayurveda and Chinese medicine [9]. Its active components include berberine, berbamine, and palmatine [10]. Today, berberine is also produced through chemical synthesis. The chloride or sulfate salt of berberine is typically used for clinical applications. Berberine has been utilized for at least 3,000 years in Ayurveda and Chinese medicine, largely due to its potent antimicrobial, antiprotozoal, and antidiarrheal properties [11]. Moreover, clinical research over time has revealed that berberine possesses a broad range of pharmacological effects. Numerous studies suggest that it exhibits significant antioxidant, anti-inflammatory, antiarrhythmic, antihypertensive, anticancer, antihyperglycemic, analgesic, antidepressant, anxiolytic, neuroprotective, and hypolipidemic activities [12-14]. Additionally, the nephroprotective [15], hepatoprotective [16,17], cardioprotective [18], and cerebroprotective [14] effects of berberine have been demonstrated in various studies. Recently, there has been an emphasis on the potential therapeutic use of berberine in ischemia-reperfusion (I/R) injury of different organs, given its notable antioxidant effect and tissue injury prevention properties. For instance, the protective effects of berberine administration have been observed in renal and testicular I/R injury in rats [19,20].
Delayed diagnosis and treatment of ovarian torsion can lead to irreversible damage to the ovaries, potentially resulting in infertility in women [21]. Recent research indicates that simply detorsing the ovary does not fully address fertility issues [22,23]. Alongside detorsion, antioxidant agents have been employed to safeguard ovarian reserves. Given this context, we aimed to determine the effects of the short-term and long-term use of berberine, which has been shown to have antioxidant effects in different studies, on ovarian I/R injuries.
Methods
Ethics approval for this study was granted by the ethics committee of Dokuz Eylul University (Protocol No. 27/2021). The research was carried out at the Experimental Animal Laboratory of Dokuz Eylul University in 2022. The subjects of the study were 28 Wistar albino female rats, each 8 weeks old and weighing between 180 and 220 g. Throughout the experimental period, the rats were maintained under standard environmental conditions (21±2 °C) and given regular access to water and food.
At the beginning of the study, vaginal smears were performed to standardize the effects of sex hormones, and only rats in estrus were included. The rats were then divided into four groups (n=28) in a random manner, as follows: The sham (S) group (n=7) involved a procedure where the abdomen was opened and closed after 1 minute, with saline being applied throughout the experiment. The torsion/detorsion (T/D) group (n=7), involved the creation of a torsion and detorsion model, with saline being applied throughout the experiment. The T/D+single dose berberine (T/D+Bb) group (n=7) underwent the establishment of a torsion and detorsion model, followed by the administration of a single dose of berberine (barium chloride, Sigma-Aldrich; cas.no:633-65-8) at 200 mg/kg intraperitoneally 30 minutes prior to detorsion [19]. No further treatment was applied post-detorsion. The T/D+15 days berberine (T/D+15Bb) group (n=7) involved the establishment of a torsion and detorsion model, followed by the administration of 150 mg/kg/day berberine via oral gavage for 15 consecutive days post-detorsion [20,24].
1. Establishment of an ovarian torsion and detorsion model in rats and berberine administration
A midline incision measuring approximately 2.5 to 3 cm was made in the lower abdomen of anesthetized rats. The ovaries were located by tracing the uterine horns. In the non-sham groups, the right and left ovarian tissue of the rats was rotated 360° clockwise and secured with vascular clamps. Following a 3-hour waiting period, detorsion was performed [25,26]. The first dose of berberine, at 200 mg/kg, was administered intraperitoneally 30 minutes prior to detorsion [19]. Subsequently, berberine was administered orally at a dosage of 150 mg/kg/day for 15 consecutive days post-detorsion [20,24]. After 15 days, all subjects were euthanized. The right and left ovaries were then removed. The left ovaries were preserved in formalin for histological examination, while the right ovaries were set aside for biochemical analysis.
2. Histopathologic evaluation
The left ovaries were preserved using formalin, dehydrated through varying concentrations of alcohol, and then embedded in paraffin. The maximum number of 4 μm thick sections were extracted from the ovaries and stained with hematoxylin and eosin. The histopathologic evaluation of ovarian tissue involved counting follicles and scoring injuries. The averages of primary, primordial, atretic secondary, and tertiary follicles were computed [27]. For injury scoring, parameters such as inflammation, vascular congestion, hemorrhage, and follicular cell degeneration were assessed. The specimens were semiquantitatively scored by examining a minimum of five microscopic fields. A scale from 0 to 3 was utilized for each parameter (0 for absent, 1 for mild, 2 for moderate, and 3 for severe) for each sample [28,29].
3. Biochemical evaluation
A biochemical evaluation was conducted by measuring the levels of transforming growth factor-beta1 (TGF-β) and smooth muscle actin (α-SMA). We utilized enzyme-linked immunosorbent assays to assess the levels of TGF-β and α-SMA, using products from BTLAB (Bioassay Technology Laboratory) (catalog numbers E1688Ra and E2330Ra).
4. Statistical analysis
SPSS version 26.0 (IBM Corp.) was employed for data analysis. The mean±standard deviation of the data were calculated for the analysis. The Kruskal-Wallis test was adopted to investigate the difference between the groups, and the Mann-Whitney U test was employed to identify from which group the difference originated.
Results
The findings from the histopathologic evaluation are depicted in Figure 1. In the S group, no pathologic changes were noted. The T/D group, however, exhibited significantly higher scores for follicular degeneration, vascular congestion, edema, hemorrhage, and inflammation (p<0.005) compared to the other groups (S, T/D+Bb, T/D+15Bb). No significant differences were observed between the T/D+Bb group, the sham group, and the T/D+15Bb group. Similarly, no significant difference was detected between the T/D+15Bb group and the T/D+Bb group. The histopathologic scores are detailed in Table 1.

Photomicrographs of ovarian tissue. (A) Sham group, (B) torsion/detorsion (T/D) group, (C) T/D+berberine (Bb) group, and (D) T/D+15Bb group (hematoxylin and eosin stain, scale bar: 100 μm). a)Follicles at different stages of development, vascular congestion (vc), hemorrhage (h), edema (o), follicular degeneration (fd), inflammation (i).
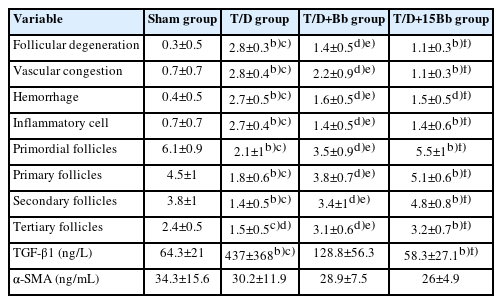
Histopathological scores of the ovaries between groups, follicular cell degeneration, vascular congestion, hemorrhage, and inflammation parametersa)
The T/D group exhibited significantly fewer primordial, primary, secondary, and tertiary follicles compared to the other groups (S, T/D+Bb, T/D+15Bb) (p<0.005). No significant difference was found between the T/D+Bb group, the sham group, and the T/D+15Bb group. Similarly, no significant difference was noted between the T/D+Bb group and the T/D+15Bb group. The follicle counts are detailed in Table 1.
Figure 2 illustrates the biochemical results of TGF-β and α-SMA levels in tissue. A statistically significant increase in the TGF-β level was observed in the tissue of the T/D group (p<0.005) compared to the other groups (S, T/D+Bb, T/D+15Bb). However, there was no significant difference between the T/D+15Bb group and the sham group. Similarly, no significant difference was found between the T/D+Bb group and the T/D group. The α-SMA levels in the tissue did not significantly differ among the groups (p>0.05).
Discussion
Recent studies involving rats have suggested that detorsion alone does not resolve fertility issues associated with ovarian torsion. Prior to the detorsion procedure, anti-inflammatory and antioxidant agents were employed to safeguard ovarian reserves. However, none of the drugs utilized in these experimental studies have been clinically approved for use in cases of ovarian torsion. Consequently, there is an ongoing search in the literature for safe and effective drugs that can be administered parenterally in humans during the brief interval between torsion and detorsion surgery [22]. Topcu et al. [30] administered a single dose of metformin prior to detorsion of the torsioned ovary. They observed a decrease in ovarian destruction, suggesting a positive contribution to treatment [30]. However, when Topcu et al. [31] administered a single dose of amiodarone before detorsion of the torsioned ovary, they did not observe a similar decrease in ovarian destruction. Other studies have recommended the long-term use of antioxidants, rather than a single dose [27,28,32]. In their research, Karakas et al. [28] demonstrated that administering metformin for 14 days protected the ovarian reserve in an ovarian T/D model. Kalyoncu et al. [32] suggested that administering octreotide shortly before and 7 days after detorsion is the most effective method for preserving the ovarian reserve in the ovarian T/D model. Our study examined the impact of a single 200 mg/kg dose of berberine administered before detorsion, and a 150 mg/kg dose of berberine given for 15 days after detorsion, on ovarian I/R injury. We aimed to compare the short-term and long-term effects of berberine administration. A high dose and peritoneal administration were chosen for immediate effects in single dose berberine administration. To observe the long-term effects, a 15-day period and medium doses were chosen for oral administration. The dosages used were determined based on a review of the literature [19,20], and it has been demonstrated that the lethal dose of oral berberine in rats exceeds 2,000 mg/kg [33].
Torsion, a condition that can affect various organs, leads to vascular congestion, edema, and hemorrhage. When torsion is corrected, or detorsed, it can result in I/R injuries. During this process, antioxidants are introduced to mitigate tissue damage caused by increased inflammation and the release of free oxygen radicals. Consequently, numerous studies have explored various pharmacological agents to prevent I/R injuries. Berberine has been identified as a potential treatment in several of these studies. For instance, Kazaz et al. [19] administered 200 mg/kg of berberine intraperitoneally 30 minutes prior to detorsion in a study of testicular I/R injury in rats, and observed protective effects. Kumas et al. [20] developed a bilateral I/R model in diabetic rats and administered varying doses of berberine (50, 100, and 150 mg/kg) over a 14-day period. They observed therapeutic effects in the groups that received 100 and 150 mg/kg doses of berberine [20]. In our study, we administered a single dose of 200 and 150 mg/kg of berberine over a 15-day period. Our histopathological evaluation, which included assessments of inflammation, follicular cell degeneration, vascular congestion, and hemorrhage, revealed reductions in the effects of I/R injuries.
Numerous pathological studies have detailed tissue damage resulting from ovarian torsion and ovarian lipid peroxidation, as well as cell death and inflammation [4-8]. Ilgen et al. [34] demonstrated that follicular degeneration, inflammatory cell infiltration, and vascular congestion were significantly elevated in the torsion group. Uzun et al. [35] conducted a comparison between ovarian torsion lasting 8 and 24 hours, finding that follicular degeneration and inflammation were more pronounced in the torsion lasting 24 hours. Consequently, the interval between torsion and detorsion surgery should be kept as brief as possible. The duration of the detorsion is thought to be extended when a conservative treatment option is pursued.
In their study, Wang et al. [36] administered berberine at doses of 100 and 200 mg/kg to rats with polycystic ovarian syndrome. They reported that this treatment could stimulate the development of antral follicles and induce ovulation [36]. In a separate study, rats with ulcerative colitis were treated with berberine. The results indicated that berberine exhibited anti-inflammatory properties by suppressing cytokines (interleukin 3 [IL-3], IL-7, IL-11, TGF-β, and tumor necrosis factor α [TNF-α]), as well as inhibiting apoptosis. These effects were found to provide a protective benefit against ulcerative colitis [37]. In our study, we corroborated the anti-inflammatory properties of berberine. Our findings showed that berberine increased the number of antral follicles, reduced inflammation as per histopathologic evaluations, and lowered TGF-β levels in the tissue.
The follicle reserve serves as an indicator of fertility. There is ongoing therapeutic research aimed at protecting the follicle reserve, which is negatively impacted by T/D injury. Eken et al. [38] observed a statistically significant reduction in the count of primordial, preantral, and antral follicles in studies related to T/D injury. They also reported a substantial increase in follicle numbers following treatment with etanercept [38]. In a separate study, berberine was administered both in vivo and in vitro in a model of premature ovarian failure. The results indicated that berberine enhanced ovarian reserve capacity and regulated ovarian hormone secretion [39]. Our study showed that 15-day supplementation of berberine increased the follicle reserve, which had been diminished due to T/D injury.
Inflammation is another pathophysiological mechanism involved in I/R injury, characterized by excessive oxidative stress resulting from reperfusion following ischemia [40]. For instance, Nayki et al. [41] demonstrated that rutin possesses antioxidative (evidenced by a decrease in malondialdehyde and cyclooxygenase activity) and anti-inflammatory (indicated by a decrease in TNF-α and IL-1β) properties against ovarian I/R injury in rats. They administered rutin 1 hour prior to detorsion. Histopathological findings revealed hemorrhage, dilated blood vessels, and degenerated follicles in the I/R group, while the rutin+I/R group exhibited nearly normal morphology [41]. In a separate study, Sagsoz et al. [4] investigated the effects of vitamin C, mannitol, and verapamil on I/R injury in rat ovaries. They observed moderate hemorrhage, edema, and loss of cohesion in the ischemia group, with more severe pathological findings in the I/R group. However, they found that vitamin C and mannitol effectively reduced I/R injuries [4]. In yet another study, it was found that rosmarinic acid modulated inflammation and prevented ovarian damage following I/R injuries [40].
During the inflammatory process, the release of platelet-derived growth factor, epidermal growth factor, and TGF-β stimulates the formation of granulation tissue, which is characterized by the presence of α-SMA-expressing myofibroblasts. TGF-β has the potential to induce myofibroblasts to overproduce extracellular matrix, which could lead to the formation of scars. If myofibroblast activity becomes excessive, it can result in fibrosis and organ dysfunction [42,43]. In the T/D group, a higher level of TGF-β was indicative of increased inflammation. Conversely, a lower level of TGF-β in the T/D+15Bb group suggested that berberine has an anti-inflammatory effect. The lack of significant differences between the T/D and T/D+Bb groups was interpreted as an indication of the weak anti-inflammatory effect of a single dose of berberine. Zhu et al. [37] demonstrated in their study that berberine possesses anti-inflammatory properties in an ulcerative colitis model. Our study further corroborates this anti-inflammatory effect. The levels of α-SMA did not significantly differ between the groups, leading us to interpret these findings as indicative of no impact on fibrosis formation in the T/D model.
In conclusion, we have demonstrated that both short- and long-term use of berberine could potentially have therapeutic effects on ovarian I/R injury. We suggest that the anti-inflammatory properties of berberine, along with its impact on follicle reserve, may aid in preserving fertility. We are confident that our study will provide a significant contribution to existing literature regarding the treatment of ovarian I/R injury. Further research will be required to determine the applicability of this agent in human subjects.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Author contributions
Conceptualization: FY, OI, AM. Data curation: FY, OI, AM, BY. Formal analysis: FY, OI, AM, SK. Funding acquisition: FY, OI, AM,SK. Methodology: FY, OI, AM, BY. Project administration: FY, OI, AM, BY, SK. Visualization: FY, BY. >Writing-original draft: FY, OI, AM, BY. riting-review & editing: FY, OI, BY, SK.

