|
 |
- Search
| Clin Exp Reprod Med > Volume 41(2); 2014 > Article |
Abstract
Objective
To study the effect of body composition on reproduction in women with unexplained infertility treated with a controlled ovarian hyperstimulation and intrauterine insemination programme.
Methods
This prospective observational study was conducted on 308 unexplained infertile women who were scheduled for a controlled ovarian hyperstimulation and intrauterine insemination programme and were grouped as pregnant and non-pregnant. Anthropometric measurements were performed using TANITA-420MA before the treatment cycle. Body composition was determined using a bioelectrical impedance analysis system.
Results
Body fat mass was significantly lower in pregnant women than in non-pregnant women (15.61±3.65 vs.18.78±5.97, respectively) (p=0.01). In a multiple regression analysis, body fat mass proved to have a stronger association with fecundity than the percentage of body fat, body mass index, or the waist/hip ratio (standardized regression coefficient≥0.277, t-value≥2.537; p<0.05). The cut-off value of fat mass, which was evaluated using the receiver operating characteristics curve, was 16.65 with a sensitivity of 61.8% and a specificity of 70.2%. Below this cut-off value, the odds of the pregnancy occurrence was found to be 2.5 times more likely.
Obesity is an important health problem that may affect reproductive functions. Obese women are at increased risk for menstrual irregularities [1], oligoanovulation, infertility, higher miscarriage rates, and complications during pregnancy [2]. Previous investigations studied the impact of obesity on the outcome of fertility treatments by using the body mass index (BMI). Some studies have suggested that the conception rate is lower in obese women than in non-obese women [3,4]. Other investigators, however, have reported either similar outcomes in obese and normal-weight women [5] or a positive effect of obesity on infertility treatment [6]. However, as individuals with the same BMI may have different body compositions, these studies have had conflicting results.
There is growing interest in the measurement of body composition, which is a simple method to measure the percentage of body fat and fat mass. Some studies investigated the importance of a body composition analysis to measure body fat mass and whether body composition can be interpreted as an extragenital marker of human ovarian function and female fertility, or not [7,8]. Since these studies included subjects who were polycystic ovary syndrome (PCOS) patients or heterogeneously stratified infertile patients, heterogeneous results were obtained; therefore, it would be more appropriate to investigate the effect of fat mass in a cohort of an unexplained infertile population. To the best of our knowledge, there has been no study on the effect of body composition on the response to cycle fecundity in a controlled ovarian hyperstimulation (COH) and intrauterine insemination (IUI) cycle. In the present report, we investigated the predictive value of body composition with respect to fecundity after COH/IUI cycles in women with unexplained infertility.
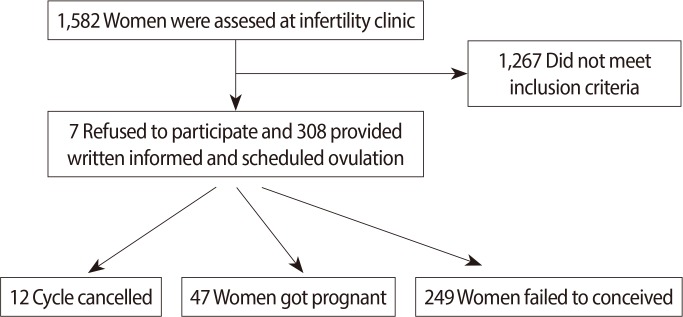
In all, 1,582 women who were admitted the infertility clinic between May 2012 and April 2013 were evaluated. Medical history, smoking habits, physical examination, level of FSH, level of luteinizing hormone, estradiol levels, semen analysis, transvaginal ultrasonography, hysterosalpingography, and chlamydial antibody titre were investigated. After the investigations, the patients who had no definable problems in terms of ovulation, oocyte reserve, tubal patency, uterine cavity, and semen analysis were diagnosed as unexplained infertility in the women and were included in this study. 1,287 patients had infertility due to some explainable factors; therefore, they were excluded from this study. The male factor was evaluated according to the World Health Organization criteria [9]. The study protocol was conducted according to the revised Declaration of Helsinki and was approved by the local Research and Ethics Committee of our hospital. As shown in the flow chart, after the exclusions, 308 pariticipants were provided written informed consent and then were scheduled for the COH/IUI protocol, 296 subjects were evaluated for the final analysis (Figure 1). The exclusion criteria encompassed all possible causes of infertility, such as endocrine factor, uterine factor in the history of surgery, pelvic inflammatory disease, presence of endometriosis, PCOS, smoking, and drug use, which might affect hormone metabolism or body composition.
Anthropometric measurements were performed using a TANITA-420MA before the treatment cycle. The analyser device was sensitive to 100 g fat. Body composition was determined using a simple and less time-consuming bioelectrical impedance analysis (BIA) system. BIA is based on the principle that the electrical conductivity through the body fluid is considerably greater in the case of fat-free mass than in the case of fat mass. Further, conventional BIA systems require four gel electrodes placed at the upper and the lower limbs, and the subject has to lie supine for the measurement. The other system that we used was a leg-to-leg BIA device that measures the impedance across the lower limbs; the four electrodes of this system were stainless steel foot pads on the top surface of a platform scale [10]. The subjects were instructed to avoid food intake for 8 hours without dehydration and with an emptied bladder. They were asked to stand barefoot on the scale for a simultaneous measurement of the body weight and the impedance; by manually entering a subject's gender and height into the system via a digital keyboard, we could immediately obtain the subject's percentage body fat on the system display. Each subject's weight, BMI (kg/m2), body fat percentage, and total body fat mass were determined using the BIA system.
BMI, which is a measure of total fatness, was classified into one of the following four BMI categories: BMI<18.5 kg/m2 (underweight), 18.5 kg/m2≤BMI<25 kg/m2 (normal weight), 25 kg/m2≤BMI<30 kg/m2 (overweight), or BMI≥30 kg/m2 (obese) [11]. Women with BMI of ≤18.5 kg/m2 or ≥30 kg/m2 were excluded.
Waist circumference was measured at the narrowest part of the waist located between the lower rib and the iliac crest, and hip circumference was measured at the level of the greatest gluteal protuberance in a horizontal plane parallel to the floor. The waist-to-hip ratio was calculated by dividing the waist circumference by the hip circumference.
All eligible subjects scheduled for the COH /IUI programme received a subcutaneous injection (75 IU per day) of exogenous gonadotropins as recombinant FSH (Puregon, Merck Serono SA, Geneva, Switzerland) beginning from the third day of the menstrual cycle. Monitoring by transvaginal ultrasound was started daily after the fifth day of the stimulus. When ≥1 follicle reached a diameter of ≥18 mm, an intramuscular injection (6,500 IU) of recombinant hCG (Ovitrelle, Merck Serono SA, Geneva, Switzerland) was administered. A concentrated, washed sperm sample was prepared, and IUI was performed using a catheter 34-36 hours after the hCG injection; the catheter was inserted through the cervical canal into the uterine cavity.
Twelve cycles were cancelled due to ovarian hyper-response. Women with delayed menses in the menstrual period following insemination were evaluated using serum hCG levels and sonograms. A positive serum beta hCG level of ≥10 mIU/L was regarded as biochemical pregnancy, and the presence of a gestational sac on ultrasonography was regarded as clinical pregnancy. At the end of this study, pregnant and non-pregnant subjects were compared in terms of their BMI, waist-to-hip ratio, and body fat mass.
All statistical analyses were performed with the SPSS ver. 15.0 (SPSS Inc., Chicago, IL, USA). Differences between the means in normally distributed variables were obtained using a Student's t-test. A chi-squared test was performed on categorical variables. Multiple regression analyses were performed to investigate the predictive value of independent variables on the occurrence of pregnancy. The strength of association between the dependent and independent variables was assessed using a standardized regression coefficient and the t-value. A receiver operating characteristics (ROC) curve was drawn to find the optimum cut-off value of the fat mass in the prediction of the pregnancy outcome.
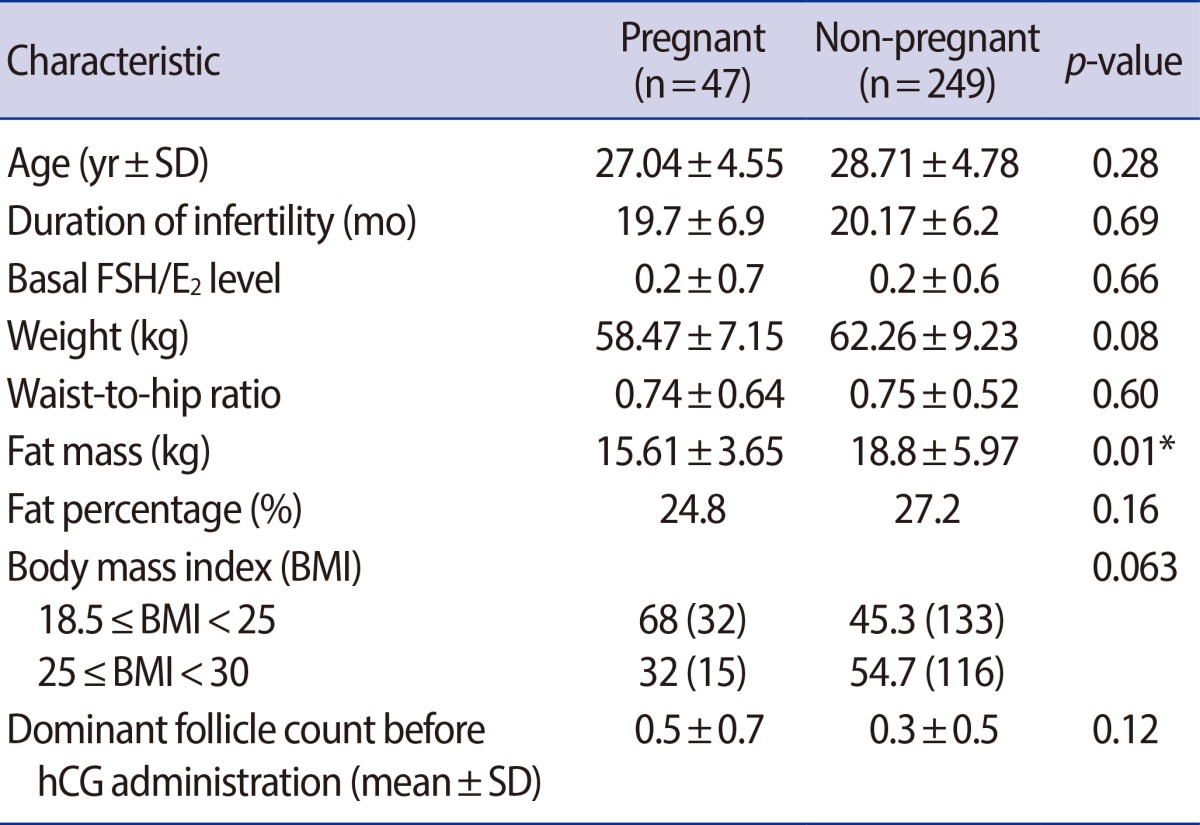
Among the 1,582 subjects, only 308 were eligible for the trial. Table 1 summarizes the demographic characteristics and anthropometry of these patients. The age, duration of infertility, basal FSH/E2 level, and dominant follicle count before the hCG administration did not differ between pregnant and non-pregnant women. The fat mass was statistically significantly lower in pregnant women than that in non-pregnant women (15.61±3.65 vs. 18.78±5.97, respectively) (p=0.01). Although the weight, waist-to-hip ratio, and fat percentage were lower in pregnant women, they were not statistically significant. Cycle fecundity was different among normal-weight and overweight women; however, this difference was also statistically non-significant (68% and 32%, respectively) (p=0.063). Women with low BMI might have the tendency to become pregnant. Note that there was only one multifetal pregnancy among the pregnant group.
In the multiple regression analysis model, fat mass proved to have a stronger association with fecundity than body fat percentage, BMI, or waist-to-hip ratio (standardized regression coefficient≥0.277; t-value≥2.537; p<0.05) (Table 2).
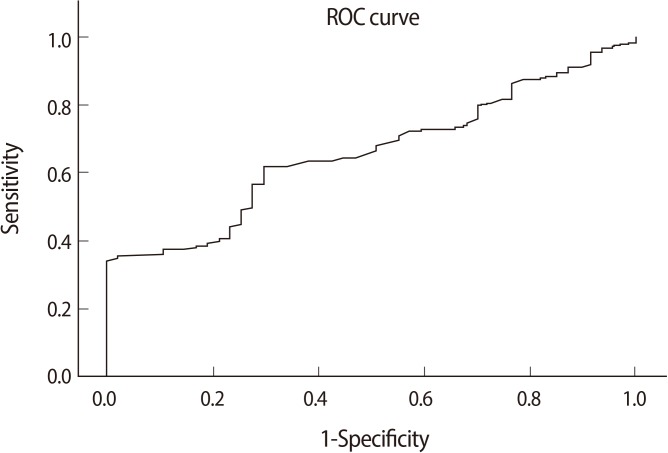
The cut-off value of fat mass was found to be 16.65 kg by using an ROC analysis with a sensitivity of 61.8% and a specificity of 70.2% (Figure 2). The area under the ROC curve was 0.65 (95% confidence interval [CI], 0.58-0.73; p=0.001). Below this cut-off value, the odds of the pregnancy occurrence was found to be 2.5 times more likely.
Our study demonstrated an association of body composition with cycle fecundity in unexplained infertile women undergoing COH/IUI. In the multiple regression analysis, body fat mass showed to be a superior predictor of fecundity to weight, BMI, and waist-to-hip ratio. The results indicate that a fat mass of >16.65 kg is a predictive factor of a pregnancy failure with a sensitivity of 61.8% and a specificity of 70.2%.
Previous studies examining the predictive value of weight with respect to fecundity by using BMI reported controversial results. Studies show similar treatment success in obese and non-obese women either undergoing COH/IUI [6,12] or IVF [5,13,14,15,16,17,18,19]. However, contrasting results of an obesity-related decline in fecundity in obese, oligoovulatory women with ovulation induction/IUI cycles were obtained in a study by White et al. [20]. Similarly, the negative impact of obesity was reported in women undergoing IVF [21,22,23,24]. In contrast, Wang et al. [6] reported a higher rate of success in obese women than in normal-weight women, but their study sample was a mixture of ovulatory and anovulatory subjects. The investigation of COH/IUI outcomes of obese women may be affected by the increased prevalence of ovulatory disorders; therefore, it is important to form a homogeneous study group to prevent selection bias. In present study, we excluded the PCOS patients because of the possibility of anovulation. Similarly, Dodson et al. [12] recruited homogenously stratified women with unexplained infertility without chronic anovulation and PCOS and reported that the adjusted cycle fecundity was not different among BMI groups: underweight, 0.14 (95% CI, 0.07-0.29); normal weight, 0.12 (95% CI, 0.09-0.16); overweight, 0.17 (95% CI, 0.12-0.24), and obese, 0.14 (95% CI, 0.08-0.23). In our study, BMI was not found to be statistically different between pregnant and non-pregnant women, but pregnant women had a tendency to have a lower BMI. We concluded that the discrepancy mentioned above, may be due to the assessment of BMI as a measurement of obesity. BMI was not a good predictor of cycle fecundity because BMI, as an indicator of the severity of obesity, measured total fatness [11] and not the body fat mass; therefore, another measurement system was required.
In some other papers, obesity was evaluated on the basis of the waist-to-hip ratio, abdominal circumference [15,25], or trunk-to-leg fat ratio [26] along with BMI. The waist-to-hip ratio, abdominal circumference, or trunk-to-leg fat ratio were found to be more predictive than BMI with respect to fecundity, particularly in studies that included PCOS and IVF patients [25,26]. The waist-to-hip ratio is the most widely used measure of fat distribution patterns [7]. Zaadstra et al. [25] reported that an increasing waist-to-hip ratio is negatively associated with the probability of conception and concluded that the body fat distribution in women of reproductive age has greater impact on fertility than age or obesity. The amount of body fat, particularly the android kind of fat distribution, is an indicator of the hormonal situation and the reproductive status of women with PCOS or anovulation. In the present study, PCOS and anovulatory patients were excluded; therefore, our results were not affected by the fat distribution. The waist-to-hip ratio describes body shape and silhouette and not the quantitative amount of body fat.
Body composition assessed using a BIA system may offer an alternative method to estimate the total body fat mass. By using body composition, the adverse health consequences of excess body fat are well documented and measurements of body fat are used in clinical practice [27]. BIA has been proven to be a simple, reliable method of assessing the total body fat mass [10]. There are many different methods to estimate the body fat mass. Hydrostatic weighing, skin-fold calipers, magnetic resonance imaging, and soft-tissue ultrasound measurement can also be used as indirect methods [28]). Dual-energy X-ray absorptiometry (DEXA) can be used as an alternative method to estimate body composition in addition to bone mineral content and density [10], but it is more expensive and the radiation exposure will have to be monitored [10]. Kirchengast and Huber [7] investigated the BMI, body weight, fat distribution patterns, and body composition of normal-weight and underweight infertile women, by using DEXA rather than a BIA system. The study reported that infertile young women revealed differences in body composition and fat distribution patterns when compared with their fertile counterparts. In our study, the fat mass seemed to have a greater impact on fecundity than the other measures of obesity.
Our study was strengthened by the homogenous group of the unexplained infertile patients. All subjects were ovulatory without hirsutism and lacked an explanatory reason for infertility.
One of the limitations of this study was its relatively small sample size. The obtained pregnancy rate was 15.9%, and the absolute sample
size for the pregnant group (n=47) was small.
In conclusion, an increasing body fat mass is negatively associated with the probability of conception per cycle; therefore, a simple method to measure the body fat mass with a BIA system will be of considerable value in clinical practice. Although the method to measure the amount of body fat was reported to be reliable, this should be experimentally validated.
References
1. Wise LA, Rothman KJ, Mikkelsen EM, Sorensen HT, Riis A, Hatch EE. An internet-based prospective study of body size and time-to-pregnancy. Hum Reprod 2010;25:253-264.PMID: 19828554.


3. Murakawa H, Hasegawa I, Kurabayashi T, Tanaka K. Polycystic ovary syndrome. Insulin resistance and ovulatory responses to clomiphene citrate. J Reprod Med 1999;44:23-27.PMID: 9987735.

4. Mitwally MF, Kuscu NK, Yalcinkaya TM. High ovulatory rates with use of troglitazone in clomiphene-resistant women with polycystic ovary syndrome. Hum Reprod 1999;14:2700-2703.PMID: 10548604.


5. Styne-Gross A, Elkind-Hirsch K, Scott RT Jr. Obesity does not impact implantation rates or pregnancy outcome in women attempting conception through oocyte donation. Fertil Steril 2005;83:1629-1634.PMID: 15950629.


6. Wang JX, Warnes GW, Davies MJ, Norman RJ. Overweight infertile patients have a higher fecundity than normal-weight women undergoing controlled ovarian hyperstimulation with intrauterine insemination. Fertil Steril 2004;81:1710-1712.PMID: 15193505.


7. Kirchengast S, Huber J. Body composition characteristics and fat distribution patterns in young infertile women. Fertil Steril 2004;81:539-544.PMID: 15037399.


8. Cosar E, Ucok K, Akgun L, Koken G, Sahin FK, Arioz DT, et al. Body fat composition and distribution in women with polycystic ovary syndrome. Gynecol Endocrinol 2008;24:428-432.PMID: 18850379.


9. World Health Organization Department of Reproductive Health and Research. Organization laboratory manual for the examination and processing of human semen. 5th ed. Geneva: World Health Organization; 2010.
10. Sung RY, Lau P, Yu CW, Lam PK, Nelson EA. Measurement of body fat using leg to leg bioimpedance. Arch Dis Child 2001;85:263-267.PMID: 11517118.



11. National Heart, Lung, and Blood Institute. North American Association for the Study of Obesity. NIH Publication 1998, No. 98-4083. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. Bethesda, MD: National Heart, Lung, and Blood Institute; 2000.
12. Dodson WC, Kunselman AR, Legro RS. Association of obesity with treatment outcomes in ovulatory infertile women undergoing superovulation and intrauterine insemination. Fertil Steril 2006;86:642-646.PMID: 16782095.


13. McClure N, McQuinn B, McDonald J, Kovacs GT, Healy DL, Burger HG. Body weight, body mass index, and age: predictors of menotropin dose and cycle outcome in polycystic ovarian syndrome? Fertil Steril 1992;58:622-624.PMID: 1521660.


14. Spandorfer SD, Kump L, Goldschlag D, Brodkin T, Davis OK, Rosenwaks Z. Obesity and in vitro fertilization: negative influences on outcome. J Reprod Med 2004;49:973-977.PMID: 15656214.

15. Wass P, Waldenstrom U, Rossner S, Hellberg D. An android body fat distribution in females impairs the pregnancy rate of in-vitro fertilization-embryo transfer. Hum Reprod 1997;12:2057-2060.PMID: 9363729.


16. Lashen H, Ledger W, Bernal AL, Barlow D. Extremes of body mass do not adversely affect the outcome of superovulation and in-vitro fertilization. Hum Reprod 1999;14:712-715.PMID: 10221701.


17. Fedorcsak P, Dale PO, Storeng R, Ertzeid G, Bjercke S, Oldereid N, et al. Impact of overweight and underweight on assisted reproduction treatment. Hum Reprod 2004;19:2523-2528.PMID: 15319380.


18. Wattanakumtornkul S, Damario MA, Stevens Hall SA, Thornhill AR, Tummon IS. Body mass index and uterine receptivity in the oocyte donation model. Fertil Steril 2003;80:336-340.PMID: 12909496.


19. Rouzi AA, Mackinnon M, McComb PF. Predictors of success of reversal of sterilization. Fertil Steril 1995;64:29-36.PMID: 7789577.


20. White DM, Polson DW, Kiddy D, Sagle P, Watson H, Gilling-Smith C, et al. Induction of ovulation with low-dose gonadotropins in polycystic ovary syndrome: an analysis of 109 pregnancies in 225 women. J Clin Endocrinol Metab 1996;81:3821-3824.PMID: 8923819.


21. Nichols JE, Crane MM, Higdon HL, Miller PB, Boone WR. Extremes of body mass index reduce in vitro fertilization pregnancy rates. Fertil Steril 2003;79:645-647.PMID: 12620460.


22. Wang JX, Davies M, Norman RJ. Body mass and probability of pregnancy during assisted reproduction treatment: retrospective study. BMJ 2000;321:1320-1321.PMID: 11090515.



23. Loveland JB, McClamrock HD, Malinow AM, Sharara FI. Increased body mass index has a deleterious effect on in vitro fertilization outcome. J Assist Reprod Genet 2001;18:382-386.PMID: 11499322.



24. Ferlitsch K, Sator MO, Gruber DM, Rucklinger E, Gruber CJ, Huber JC. Body mass index, follicle-stimulating hormone and their predictive value in in vitro fertilization. J Assist Reprod Genet 2004;21:431-436.PMID: 15704518.



25. Zaadstra BM, Seidell JC, Van Noord PA, te Velde ER, Habbema JD, Vrieswijk B, et al. Fat and female fecundity: prospective study of effect of body fat distribution on conception rates. BMJ 1993;306:484-487.PMID: 8448457.



26. Douchi T, Oki T, Yamasaki H, Nakae M, Imabayashi A, Nagata Y. Body fat patterning in polycystic ovary syndrome women as a predictor of the response to clomiphene. Acta Obstet Gynecol Scand 2004;83:838-841.PMID: 15315595.


27. Jebb SA, Cole TJ, Doman D, Murgatroyd PR, Prentice AM. Evaluation of the novel Tanita body-fat analyser to measure body composition by comparison with a four-compartment model. Br J Nutr 2000;83:115-122.PMID: 10743490.


28. Vehrs P, Morrow JR Jr, Butte N. Reliability and concurrent validity of Futrex and bioelectrical impedance. Int J Sports Med 1998;19:560-566.PMID: 9877148.