Non-invasive evaluation of embryo quality for the selection of transferable embryos in human in vitro fertilization-embryo transfer
Article information
Abstract
The ultimate goal of human assisted reproductive technology is to achieve a healthy pregnancy and birth, ideally from the selection and transfer of a single competent embryo. Recently, techniques for efficiently evaluating the state and quality of preimplantation embryos using time-lapse imaging systems have been applied. Artificial intelligence programs based on deep learning technology and big data analysis of time-lapse monitoring system during in vitro culture of preimplantation embryos have also been rapidly developed. In addition, several molecular markers of the secretome have been successfully analyzed in spent embryo culture media, which could easily be obtained during in vitro embryo culture. It is also possible to analyze small amounts of cell-free nucleic acids, mitochondrial nucleic acids, miRNA, and long non-coding RNA derived from embryos using real-time polymerase chain reaction (PCR) or digital PCR, as well as next-generation sequencing. Various efforts are being made to use non-invasive evaluation of embryo quality (NiEEQ) to select the embryo with the best developmental competence. However, each NiEEQ method has some limitations that should be evaluated case by case. Therefore, an integrated analysis strategy fusing several NiEEQ methods should be urgently developed and confirmed by proper clinical trials.
Introduction
Over the past half-century, strategies have been developed to evaluate and select competent preimplantation embryos for uterine transfer in human in vitro fertilization-embryo transfer (IVF-ET) programs. In the early days, morphological characteristics including fragmentation and other features of embryos observed by an optical microscope were mainly used to evaluate the quality and developmental potential of embryos [1]. However, simple daily microscopic observations by clinical embryologists had limitations in accurately predicting the developmental capacity of embryos.
Recently, techniques for efficiently evaluating the state and quality of embryos using time-lapse monitoring systems (TLMSs) and various molecular genetic approaches have been introduced. In particular, TLMS could select viable embryos without concerns regarding observer-variability and disturbances of culture conditions [2]. Various studies have searched for optimal morphokinetic parameters during TLMS, which could enhance the probability of blastocyst formation, aneuploidy, and finally implantation. Analyses of implantation-related morphokinetic parameters during TLMS have facilitated the development of several clinical algorithms as promising tools for the evaluation and prediction of embryos destined to become the most competent blastocysts [3].
Artificial intelligence (AI) programs based on deep learning technology and big data analysis of TLMS have been developed and applied as a method for the non-invasive evaluation of embryo quality (NiEEQ). The clinical effectiveness of NiEEQ for human IVF-ET programs has just begun to be reported, and there are several algorithms to predict the implantation potential of day-3 or day-5 embryos. Although the application of NiEEQ alone may not be perfect for selecting the best embryos, more advanced information about the physiological and genetic state of embryos could provide insights into all aspects of the embryos’ intrinsic characteristics [4].
Furthermore, advanced and sensitive molecular genetic approaches have been successfully applied to spent embryo culture media (SECM), which can be easily obtained during in vitro embryo culture. It is possible to analyze small amounts of cell-free DNA (cfDNA), mitochondrial DNA, microRNA, and long non-coding RNA secreted from embryos using real-time polymerase chain reaction (PCR) or digital PCR, as well as next-generation sequencing (NGS) [5-7]. In addition, studies have evaluated the developmental ability of embryos by analyzing substrates and metabolites produced during in vitro culture, which will be discussed further below [8-11]. Many recent studies have evaluated the correlation between the results of SECM analysis and the embryos’ developmental competence. However, the results obtained from those methods can be affected by various external sources of contamination and have the disadvantages of needing relatively expensive equipment, having high costs, and requiring special expertise.
This review provides an overview of the current status of NiEEQ, including TLMS and advanced molecular biological methods in SECM analysis. We also describe the need to develop a method for integrated analysis to overcome the several limitations of each NiEEQ system that has been used in recent years.
TLMS for the selection of the best embryos for transfer
In human IVF-ET programs, embryo cleavage is observed daily by microscopy during in vitro culture, and the quality of embryos is determined by the number of blastomeres, cell symmetry, percentage of fragmentation, and other parameters on the day of transfer. The quality of blastocysts is also assessed according to the blastocysts’ expansion state and the appearance of the inner cell mass (ICM) and trophectoderm cells (TE) [12]. Transferable embryos are traditionally selected through a time-point observation of morphological features by trained clinical embryologists with expertise in embryo evaluation [13]. However, there are some limits in accurately predicting the developmental capacity of embryos by microscopic observations. Inter- and intra-observer variations can occur in embryo grading, even when it is performed by expert clinical embryologists [14].
For this reason, the TLMS was developed and applied in human IVF-ET programs [15]. A time-lapse system allows the complete observation of developing embryos in the IVF laboratory within stable culture conditions [16]. Initially, valuable knowledge was obtained through the TLMS during in vitro culture of pre-implantation embryos in animal models, such as mice and cows, and the TLMS provided precise information on developmental dynamics by making it possible to recognize important morphological changes of the embryo state [17,18]. The advantages of this system include a reduced need for handling and human risk, uninterrupted culture conditions, the ability to detect abnormal events that would otherwise not be noticed, and reduced inter- and intra-observer variability [16,19].
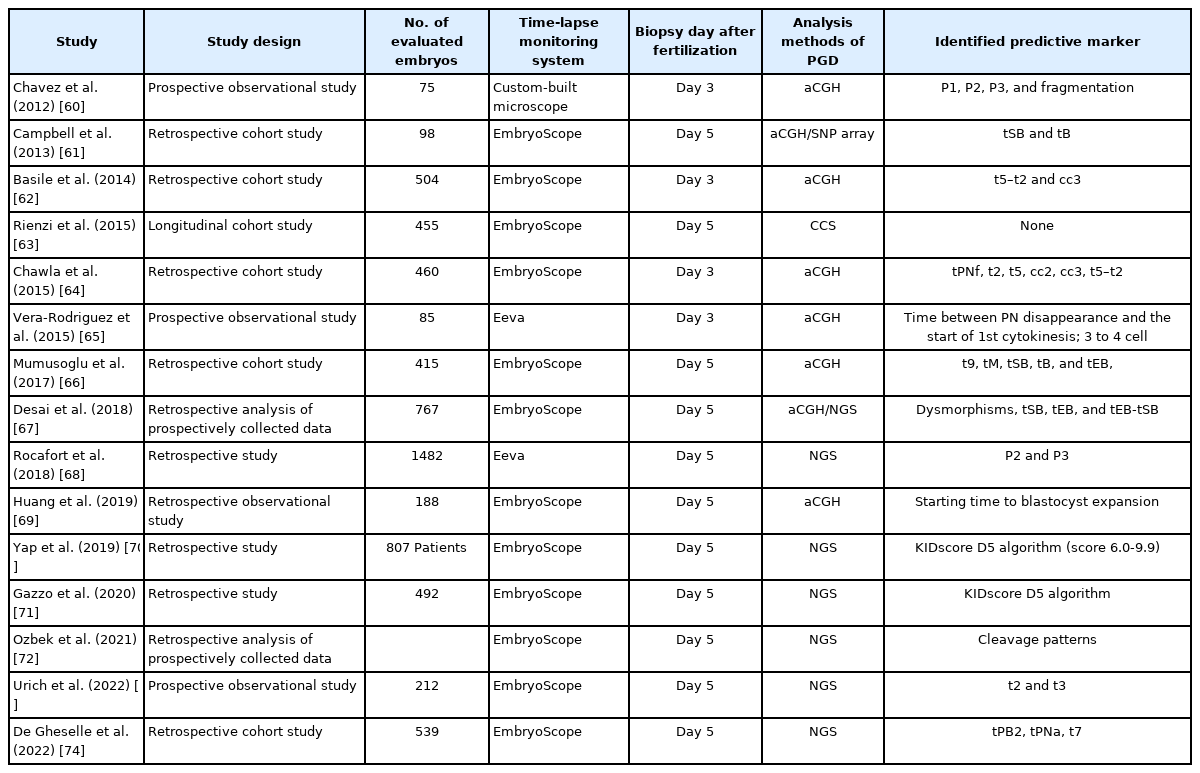
Through TLMS, various morphokinetic markers of developing embryos to predict blastocyst formation have been proposed, as shown in Table 1 [20-35]. In addition to blastocyst formation, morphokinetic markers associated with embryo implantation have been identified, as shown in Table 2 [22,24,25,34,36-50]. Several algorithms using a combination of morphokinetic variables have been introduced and successfully applied in human IVF-ET to select embryos with higher developmental capacity and implantation potential.
The known implantation data (KID) score is an interesting algorithm to improve embryo selection and predict implantation and live birth. The KID score algorithm attempts to rank embryo quality and optimize embryo selection prior to transfer based on conventional morphology grading [51]. Several reports have demonstrated the efficacy of the KID score algorithm and other similar programs using AI. The KID scores of day-5 blastocysts were found to be inversely proportional to maternal age, but directly proportional to blastocyst morphological grade [52]. This finding indicates that the KID score model works well to select blastocysts with higher implantation potential in patients with advanced maternal age.
More recently, the iDAScore algorithm has been developed; this is a deep learning-based annotation scoring system to predict the viability of embryos and the likelihood of implantation and pregnancy. Automatic embryonic ranking systems with AI have demonstrated higher performance with respect to successful implantation and pregnancy prediction than conventional morphological grading systems for the selection of transferable embryos [53]. The area under the curve (AUC) for the iDAScore was comparable to or higher than those of the KID score and Gardner criteria for young and older groups. In particular, for younger women, the AUC of iDAScore was 0.72, which was greater than those of the other two models.
For the KID score, strongly predictive morphokinetic variables were identified (time to 2 cells, duration of the second cell cycle below or above a threshold) with regard to implantation and live birth following IVF in a retrospective study comprising 2,827 embryos [54]. For the AI-based automated iDAScore, two parameters were identified (blastocyst grading and direct cleavage) using retrospective data from 18 IVF clinics consisting of 115,832 embryos, of which 14,644 embryos were assessed using the KID score [55].
Another AI-based model, termed Life Whisperer (LW), was developed by assessing the images of 8,886 embryos from 11 IVF clinics and provided time-saving and higher accuracy for successful pregnancy [56]. The LW model significantly improves the predictive accuracy of embryologists for viable and non-viable embryos. The weighted overall accuracy was 64.3% for embryo viability, with an improvement of 24.7% over embryologists’ accuracy. This model showed a sensitivity of 70.1% and specificity of 60.5% for viable embryos, while still showing a bias toward high sensitivity.
The DynScore, constructed in 2021, is a model calculated with the Gaussian distributions of the "a" coefficients (defined as the estimated number of maximum cells at 72 hours equivalent to the asymptote of the logistic curve). Logistic regression was performed using morphokinetic parameters from the first 3 days of 1,186 embryos, and the model output was highly predictive of blastocyst formation, with an AUC above 0.9 [57]. Although this model used a machine learning system with reinforcement capacity to predict the fate of embryos, it was only useful for specific types of patients, and it was not able to predict pregnancy.
Deep learning models have achieved good prediction results for successful pregnancy and fetal heartbeat following selected blastocyst transfer [58,59], and a few studies have reported the prediction of embryo euploidy [60-62]. The correlation between euploidy and embryo morphokinetics has been widely studied, as shown in Table 3 [60-74]. Using a known data set of single static embryo images, the Embryo Ranking Intelligent Classification Algorithm was developed to rank embryos based on ploidy and implantation potential [75]. The euploid prediction algorithm, with comprehensive consideration of morphokinetic parameters, patient age, and ploidy state determined by preimplantation genetic testing (PGT) improved the predictive efficiency and accuracy (the AUC of 0.80) [76]. To improve the benefits of the AI model in terms of prediction of clinical pregnancy, validation for AI applications with a large-scale dataset is needed. The morphological features of embryos are not absolute, and do not fully represent the potential of embryos for successful implantation.
Despite their widespread application, embryo morphological assessments with TLMS have limited predictive power, especially for genetic variations and metabolic competence. Several studies have clearly demonstrated that embryo morphology and time to blastocyst formation are linked to embryo metabolism. Many approaches rely on intracellular measurements or quantification of metabolites in the spent media to detect the metabolic activity of the whole embryo. Those methods are either invasive or require highly specialized skills. Non-invasive techniques to measure embryonic development and metabolic state may assist in improving embryo selection in clinical laboratories.
Development and advancement of molecular biological methods for the analysis of SECM
Traditionally, invasive biopsy of pre-implantation embryos is performed for PGT to identify inherited or de novo euploidy or aneuploidy (Figure 1A). However, aneuploid cells are preferentially eliminated from mosaic embryos via processes of apoptosis or expulsion of cells during compaction. This is a cause of misdiagnosis or poor pregnancy outcomes. It was found that autophagy-mediated apoptosis eliminated aneuploid cells in a mouse model of chromosome mosaicism [77].
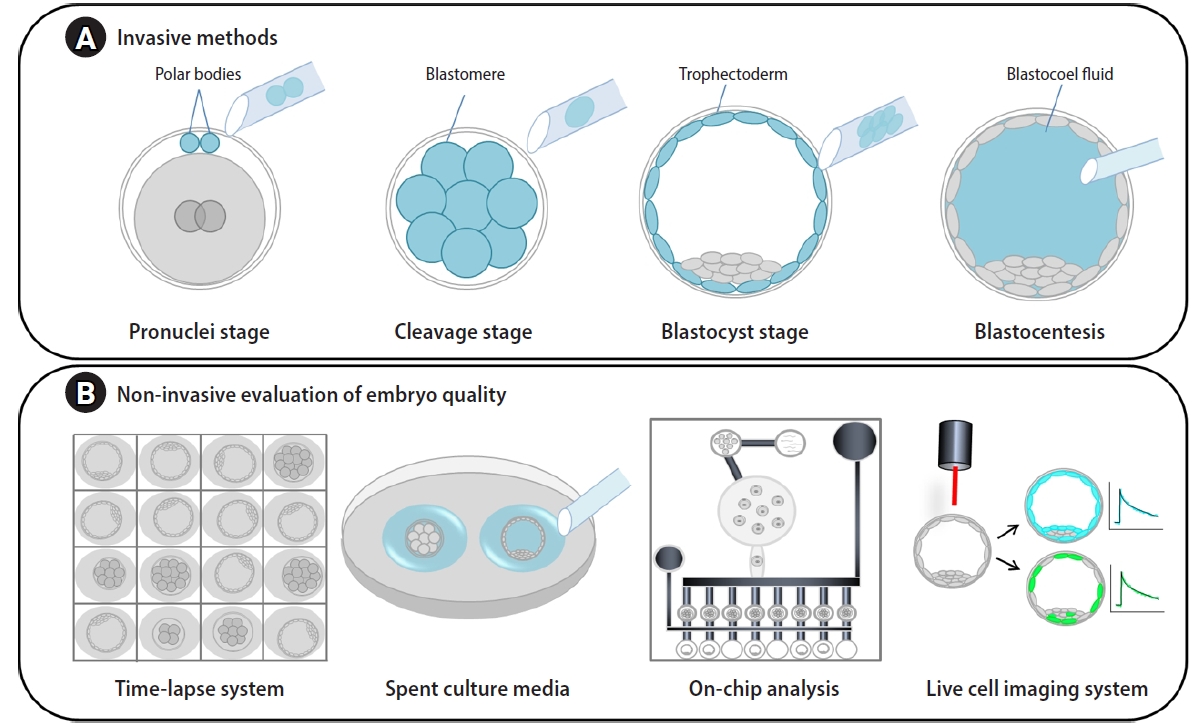
Invasive and non-invasive methods for evaluation of embryo quality. (A) For invasive methods, genetic materials can be obtained by polar body biopsy at the pronuclei stage, blastomere biopsy for cleavage-stage embryos, and trophoblast biopsy or blastocoel fluid aspiration for blastocyst-stage embryos. (B) For non-invasive methods, image analysis of embryos using time-lapse monitoring systems; spent culture media analysis with quantitative real-time polymerase chain reaction (PCR), digital PCR, and on-chip-analyses; and live cell image analyses using advanced microscopic systems have been applied.
Many researchers have used non-invasive methods to determine the metabolic and genetic state of embryos concerning their viability and pregnancy outcomes for IVF patients (Figure 1B). During in vitro culture of human embryos, a variety of macromolecules, including proteins, nucleic acids, genetic material, and extracellular vesicles are present in SECM. Of many molecules, the level of GDF9 in human SECM was linked to embryo quality and viability [78]. Interestingly, various miRNA populations have been detected in the SECM, and these miRNAs may influence genes impacting early embryo development [79]. Profiling the secretome in SECM provides potential diagnostic biomarkers for embryo quality and ploidy [80-82]. Interestingly, a comparative analysis of the metabolomic profiles of SECM on day 5 found two different clusters of metabolite composition between euploid and aneuploid embryos with good morphology. Furthermore, untargeted metabolomics of SECM by high-performance liquid chromatography–mass spectrometry identified potential biomarkers of embryos with good morphology that would undergo unsuccessful implantation [83]. In a preliminary report, three artificial neural networks that combined morphological variables and proteins using blastocyst image analysis and proteomic profiles of SECM were able to predict live birth, with an AUC of 1.0 in receiver operating characteristic curve analysis [84]. The researchers suggested that their model may provide an efficacious tool to select the embryo most likely to lead to a live birth in a euploid cohort. It may be applied to reduce the number of transferred embryos per patient to prevent complicated multiple pregnancies.
The reported levels of ploidy agreement between non-invasive SECM samples and biopsied embryonic cells vary widely [85]. A study found various cfDNAs in SECM from 57 embryos of seven IVF patients, and their genetic testing by array-based comparative genomic hybridization was consistent with TE biopsy [86]. Furthermore, single-cell bisulfite sequencing of SECM identified cfDNAs derived from human blastocysts, cumulus cells, and polar bodies, and detected cellular origin and chromosome aneuploidy. The DNA methylation-based approach decreases the risk of contamination by maternal components, which interfere with a genetic diagnosis [87].
The greatest advantage of non-invasive genetic testing is cost-effectiveness due to the lack of fees for embryo biopsy, and it is useful as first-line PGT [88,89]. The efficiency of this method has been restricted by technical complications associated with DNA contamination and low sensitivity, resulting in clinical misdiagnoses [90]. In many cases, a small sample size reduces the reliability of the results of non-invasive PGT. Larger-scale and well-designed studies testing embryo-derived and extra-embryonic genetic material are warranted to shed light on the mechanisms and potential dynamics of embryo mosaicism.
Another issue to be considered for non-invasive genetic testing is SECM preparation. Group culture is not suitable, and it is necessary to place only one embryo in each small drop of culture medium. This aspect strongly affects culture conditions by evaporation and leads to excessive use of culture dishes. Modification of the culture platform on which gametes, embryos and media flow are handled may offer benefits including rapid fluid manipulation and feasibility of usage. The microfluidic method utilizes fluid movement along microchannels in a micro- or nano-environment during cell culture, while the embryos remain largely undisturbed [91]. Microfluidics platforms facilitate the easy manipulation or removal of gametes/embryos dealing with small volumes and the examination of metabolomic activity and profiles, offering a feasible non-invasive predictor of embryo quality [91,92]. Some lab-on-a-chip devices have met with a certain degree of success in adherent cell systems [93,94]. The technical development of integrative automation for more complex procedures within the same platform remains a work in progress. Embryo culture and subsequent analysis on the same platform offer the ability to reduce cell handling and the potential introduction of laboratory errors.
Electron carriers, such as nicotinamide adenine dinucleotide and flavin adenine dinucleotide, have recently been used to characterize variations in the metabolic state obtained using fluorescence lifetime imaging microscopy (FLIM) [95]. This measuring system allows the observation of distinct metabolic states between ICM and TE, and makes it possible to detect variations in individual blastocysts from the same patient and between patients. However, the association between FLIM data and embryo ploidy has not yet been fully elucidated.
Clinical outcomes of TLMS and SECM analysis in human IVF-ET programs
TLMS for pre-implantation embryos in human IVF-ET programs provides more embryo information as a non-invasive tool. However, it has been debated whether using a TLMS could improve the clinical outcomes compared with conventional evaluation systems. These TLMSs have been applied to clinical practice since the early 2000s, and many clinical trials have been reported. This review discusses the overall trend and future directions through a review of meta-analyses of clinical trials.
The first meta-analysis on the efficiency of TLMS was reported in 2014 [96]. The authors suggested that TLMS does not significantly offer the likelihood of achieving clinical and ongoing pregnancy in blastocyst transfer. They concluded that more research is needed to improve the quality of the available evidence and to investigate the usefulness of TLMS interventions for the selection of transferable embryos.
Thereafter, several meta-analyses were published until 2019, and all suggested that it is difficult to confirm a significant difference between TLMS and conventional methods [97-100]. In a Cochrane review published in 2019, the authors concluded that there was insufficient good-quality evidence of differences in live birth, ongoing pregnancy, miscarriage and stillbirth, and clinical pregnancy rates between TLMS and conventional methods.
However, a recent meta-analysis reported that TLMS interventions were effective [101]. Two randomized controlled clinical trials demonstrated the efficacy of TLMS in various conditions in the last 6 years [102,103]. Many retrospective studies using TLMS have reported statistically significantly higher rates of pregnancy success compared to traditional methods [54,104-107]. In addition, the KID score and iDAScore, using AI algorithms based on deep learning, have been developed and their applications are expanding in human IVF-ET programs [3,51,53,108,109]. The fully automated iDAScore model reduces manual evaluation and eliminates bias due to inter- and within-observer variability [55].
However, a couple of studies have reported that the evidence for significant advantages of TLMS remains unclear [110-112]. Elective single cleavage-stage embryo transfer with TLMS did not have any advantages over conventional observation in young women with good ovarian reserve [111]. That study also suggested that single blastocyst transfer with TLMS does not increase the likelihood of ongoing pregnancy compared to conventional observation; in particular, the use of a TLMS to choose blastocysts for fresh single embryo transfer on day 5 did not improve ongoing pregnancy rate compared to morphology alone [112].
One of the important challenges in the field of PGT of preimplantation embryos is the use of non-invasive procedures [113-115], with the aim of improving PGT cost-efficiency and safety. The collection of SECM is not a difficult procedure, and it can be safely performed on all cultured embryos. It does not require special expertise in embryo manipulation, unlike invasive biopsies of embryos and blastocysts. Since this method avoids all detrimental effects of suboptimal micromanipulations and the potential risks caused by invasive procedures, it does not affect the embryo development and reproductive potential. Moreover, SECM can be collected at any pre-implantation developmental stage; even cleaved embryos with fewer than six cells on day 3 and early blastocysts can be tested, unlike invasive PGT, which is based on embryo biopsy or blastocentesis. Hence, PGT by SECM might be particularly suitable for cultured growing embryos with low implantation potential that cannot be tested by invasive PGT. In fact, SECM–PGT is relatively fast, taking less than 12 hours from SECM collection to genetic analysis [116-118], and the results may be available before embryo transfer or cryopreservation. If there is a positive diagnosis, another SECM sample should be collected after 24 hours of incubation for confirmation. Many published reports have suggested that SECM is a potential alternative source of embryonic DNA, indicating that SECM-PGT is a promising procedure for the genetic testing of all developing embryos [119,120]. However, before implementing SECM-PGT in clinical practice, it is necessary to improve its reliability [121]. The standardization of SECM-PGT and establishment of guidelines are also essential to enable reliable comparisons of results and to verify the consistency of results among IVF-ET centers.
Debate continues regarding the reliability of alternative sources of genetic materials for embryo evaluation, although cfDNAs from SECM have been successfully detected and amplified. Discrepancies have been found regarding the concordance of the embryonic genetic state obtained from SECM and other DNA sources, including polar bodies, embryos, and TE biopsies, and whole embryos. There have been many discussions and suggestions on standardization and validation methods in several review papers on this issue [90,113,122-125]. With the latest advanced methods, such as NGS and mass spectrometry, which have recently emerged as superior analysis methods, it has become possible to verify the source of the genetic sample used for analysis and assess the probability of accurately estimating the genetic state of the embryo [126]. However, mosaicism, multinucleation, blastomere fragmentation, and contamination of SECM are still difficult to overcome. In particular, it is not easy to accurately distinguish genetic material of maternal or paternal origin and from the embryo.
Conclusion
The ultimate goal of human IVF-ET programs is to achieve a healthy pregnancy and birth, ideally from the selection and transfer of the single best, most competent embryo. The effectiveness of NiEEQ in clinical applications of human IVF-ET programs has been pursued intensively. However, each non-invasive method, such as TLMS and SECM analysis, has limitations that must be handled case by case. Since the evaluation of the embryo state using TLMS is based on morphological criteria, it is impossible to confirm variation at the actual genome and gene expression level. It is also difficult to reflect differences according to the culture conditions of each laboratory and the characteristics of each individual. In SECM analysis, alterations of nucleic acids and metabolites may appear depending on the presence or absence of cumulus cells or sperm that can be cultured if they attach to the fertilized oocytes. In order to overcome these limitations of NiEEQ, it would be ideal to develop integrated analysis methods through the fusion of complementary methods.
Rapidly developing, deep learning and AI algorithms with big data analysis can play a crucial role in improving and assisting many methods of both TLMS and SECM analysis. Several studies are being conducted to support the application of various techniques by developing automated annotation programs for the morphological dynamics of TLMS and genetic analysis of SECM. This advanced computational approach is expected to provide fast, robust, and reliable results while reducing bias in the interpretation of data and selection of the best embryo.
In the near future, it is expected that new integrated NiEEQ methods will emerge that can combine the advantages and compensate for the disadvantages of these two methods. We need to develop an integrated NiEEQ for the best embryo selection to achieve a healthy pregnancy and birth.
Notes
Conflict of interest
Jin Hyun Jun is an editorial board member of the journal but was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Author contributions
Conceptualization: JHJ. Data curation: JK, JL. Formal analysis: JHJ, JK. Funding acquisition: JL, JHJ. Methodology: JK, JL. Visualization: JHJ, JK. Writing–original draft: JK. Writing–review & editing: JHJ, JL.