Efficacy of ablation and sclerotherapy for the management of ovarian endometrioma: A narrative review
Article information
Abstract
Ovarian cystectomy is the preferred technique for the surgical management of ovarian endometrioma. However, other techniques such as ablation or sclerotherapy are also commonly used. The aim of this review is to summarize information regarding the efficacy of ablation and sclerotherapy compared to cystectomy in terms of ovarian reserve, the recurrence rate, and the pregnancy rate. Several studies comparing ablation versus cystectomy or sclerotherapy versus cystectomy in terms of the serum anti-Müllerian hormone (AMH) decrement, endometrioma recurrence, or the pregnancy rate were identified and summarized. Both ablation and cystectomy have a negative impact on ovarian reserve, but ablation results in a smaller serum AMH decrement than cystectomy. Nonetheless, the recurrence rate is higher after ablation than after cystectomy. More studies are needed to demonstrate whether the pregnancy rate is different according to whether patients undergo ablation or cystectomy. The evidence remains inconclusive regarding whether sclerotherapy is better than cystectomy in terms of ovarian reserve. The recurrence rates appear to be similar between sclerotherapy and cystectomy. There is not yet concrete evidence that sclerotherapy helps to improve the pregnancy rate via in vitro fertilization in comparison to cystectomy or no sclerotherapy.
Introduction
Endometriosis typically presents as three types: superficial peritoneal lesions, deep infiltrating endometriosis, and ovarian endometrioma [1]. Of these types, ovarian endometrioma can be easily identified by ultrasonography; it is lined with endometrial tissue and contains a chocolate-colored fluid that arises from the accumulation of menstrual debris. Ovarian endometrioma accounts for 17% to 44% of all cases of endometriosis [2]. Lee et al. [3] analyzed 1,374 cases (1,350 women) confirmed as endometriosis by pathological reports during surgery performed at a single center for 9 years. The predominant location of endometriosis was in the ovaries (96.4%), followed by soft tissues (2.8%), the gastrointestinal tract (0.3%), and the urinary tract (0.2%). In ovarian endometrioma, unilateral lesions accounted for about two-thirds of cases, and bilateral lesions for about one-third.
In symptomatic women with ovarian endometriomas, a surgical approach is usually recommended. There are three main surgical techniques: cystectomy, ablation, and sclerotherapy. The degree of symptom relief and recurrence rate should be considered when assessing the therapeutic effects of various techniques. In addition, and more importantly, the preservation of ovarian reserve and the subsequent pregnancy rate should be considered, especially in women who desire pregnancy in the future.
The aim of this review is to summarize information regarding the efficacy of ablation and sclerotherapy compared to cystectomy in terms of preservation of ovarian reserve, the recurrence rate, and the pregnancy rate.
Ovarian cystectomy
Ovarian cystectomy is the preferred technique in terms of recurrence and the spontaneous pregnancy rate after surgery [4,5]. However, cystectomy often causes ovarian damage and diminished ovarian reserve. At 9 to 12 months after ovarian cystectomy, 39.5% and 57% reductions in serum anti-Müllerian hormone (AMH) levels were observed in patients with unilateral and bilateral endometriomas, respectively [6]. Since ovarian endometrioma consists of a pseudocapsule, cystectomy leads to the removal of the lining of endometrial tissues as well as the normal ovarian tissues [7]. Furthermore, the remaining normal ovarian tissues are usually coagulated for bleeding control, thereby further diminishing ovarian reserve. A greater decline in ovarian reserve could occur in older women and those with larger ovarian endometriomas, bilateral lesions, and advanced-stage disease [6,8-11]. Therefore, cystectomy has to be chosen very carefully in women who desire future pregnancy or who are infertile. Cystectomy is a very difficult option to choose in women who already have a diminished ovarian reserve before surgery, and even in women with recurrent endometrioma after surgery. As a way to preserve ovarian reserve when cystectomy is performed, hemostasis by ovarian suturing or a hemostatic agent has been introduced.
Table 1 lists 11 comparative studies on serum AMH decrement (3 months or more postoperatively) after cystectomy of ovarian endometrioma with bipolar coagulation versus suturing (five studies), as well as bipolar coagulation versus a hemostatic agent (six studies). Although Baracat et al. [12] summarized comparative studies on serum AMH decrement after ovarian cystectomy, the meta-analysis included several studies that enrolled both endometrioma and non-endometrioma groups. Therefore, in this review, three studies that enrolled mixed groups were not included in Table 1 [13-15]. However, the study by Kang et al. [16] was included, because the serum AMH decrement in the endometrioma group could be extracted separately. The serum AMH decrement was calculated as follows: [(postoperative AMH level–preoperative AMH level)]/preoperative AMH level]×100 (%).

Comparative studies on serum AMH decrement (3 months or more postoperative) after cystectomy for ovarian endometrioma with bipolar coagulation versus suturing and bipolar coagulation versus a hemostatic agent
Among the five studies comparing bipolar coagulation versus suturing, the serum AMH decrements were similar in three studies [17-19]. However, two studies reported significantly smaller serum AMH decrements in the suturing group [20,21]. Among the six studies comparing bipolar coagulation versus hemostatic agent, the serum AMH decrements were similar in three studies [22,23,24]. However, three studies reported significantly smaller serum AMH decrements in the hemostatic agent group [16,25,26].
Interestingly, Araujo et al. [24] compared serum AMH decrements after all three methods (bipolar coagulation versus suturing versus a hemostatic agent), but the serum AMH decrements were similar in all groups. These 11 studies showed varying results for the results of the serum AMH decrement; therefore, no conclusions can be drawn. Thus, it remains unclear whether suturing or the use of hemostatic agents as a method of hemostasis results in smaller serum AMH decrements compared to bipolar coagulation.
In a systematic review and meta-analysis, 3-month postoperative AMH levels were significantly lower in patients who received bipolar coagulation group than in those for whom a non-thermal hemostasis method was used (mean difference, –0.79 ng/mL; 95% confidence interval [CI], –1.19 to –0.39) [27]. In that report, only three studies were included; in one study, 3-month postoperative AMH levels were compared between bipolar coagulation versus a hemostatic agent [22], while two studies compared 3-month postoperative AMH levels between bipolar coagulation and suturing [19,21].
Ablation versus cystectomy
Ablation is a method of incising an ovarian endometrioma to remove the internal fluid and ablate the lining of endometrial tissue. Ablation can be performed using bipolar coagulation, laser vaporization, or plasma energy [28]. Since the cyst wall is not removed, it is generally considered a better option than cystectomy in terms of ovarian reserve [11]. Table 2 lists 11 comparative studies on serum AMH decrement, recurrence of endometrioma, or the pregnancy rate after ablation versus cystectomy of ovarian endometrioma [8,10,29-37]. In most studies, bipolar coagulation was used as a method for ablation, but laser vaporization was used in four studies [10,29-31]. Plasma energy was used in only one study [32]. It is difficult to draw a definitive conclusion from these 11 studies on which ablation technique would be better. The reader should refer to each article for details on how to use a specific ablation technique.

Comparative studies on serum AMH decrement, recurrence of endometrioma, and the pregnancy rate after ablation versus cystectomy for ovarian endometrioma
1. Serum AMH decrement
A randomized study by Giampaolino et al. [8] indicated that both ablation and cystectomy had a negative impact on ovarian reserve. However, they found that endometrioma size was associated with the magnitude of AMH decrement after ablation or cystectomy. In 24 women with endometriomas measuring <5 cm, the degree of AMH decrement at 3 months was similar between ablation and cystectomy (–18.2% vs. –17.6%), but in 22 women with endometriomas ≥5 cm in size, a smaller decline of serum AMH level was noted in the ablation group (–14.8% vs. –24.1%, p<0.05). Therefore, in cases with endometriomas ≥5 cm in size, ablation might be better than cystectomy for preserving serum AMH levels.
Another randomized study by Candiani et al. [30] indicated that ablation was better than cystectomy in terms of preserving serum AMH levels. In the ablation group, the mean preoperative and 3-month postoperative serum AMH levels were 2.3 and 1.9 ng/mL, respectively (p>0.05), while in the cystectomy group, the corresponding levels were 2.6 and 1.8 ng/mL, respectively (p<0.05). A prospective study by Saito et al. [10] showed that ablation was better than cystectomy in terms of ovarian reserve, especially in bilateral lesions =. In women with bilateral lesions, the 1-, 6-, and 12-month postoperative AMH decrements were significantly smaller in the ablation group than in the cystectomy group. However, in women with unilateral lesions, the AMH decrements were similar between the ablation and cystectomy groups.
A randomized study by Shaltout et al. [33] demonstrated that the 6-month postoperative AMH decrement was significantly smaller in the ablation group than in the cystectomy group. Interestingly, they found that the insertion of oxidized regenerated cellulose (Surgicel; Ethicon, Somerville, NJ, USA) inside the cavity of the cyst significantly minimized the AMH decrement in the ablation group, but not in the cystectomy group. A retrospective study by Chen et al. [34] indicated that both ablation and cystectomy had a negative impact on ovarian reserve, but they found that ablation was better than cystectomy in terms of ovarian reserve. In the ablation group, the mean preoperative and 6-month postoperative serum AMH levels were 4.47 and 3.95 ng/mL, respectively (p<0.05), while the corresponding levels in the cystectomy group were 4.25 and 3.40 ng/mL, respectively (p<0.05). The mean change in AMH levels was significantly smaller in the ablation group (mean, –0.52 ng/mL vs. –0.85 ng/mL, p<0.05).
In summary, five studies indicated that both ablation and cystectomy had negative impacts on ovarian reserve; however, smaller decrements in the serum AMH level after ablation were uniformly reported [8,10,30,33,34]. Ablation appears to be advantageous in terms of the preservation of ovarian reserve, especially in women with endometriomas ≥5 cm in size or bilateral lesions [8,10].
2. Recurrence rate
Seven studies compared the recurrence rate of endometrioma between ablation versus cystectomy [10,29,31,33-36]. Interestingly, five studies reported a higher recurrence rate in the ablation group than in the cystectomy group, but without a statistically significant difference [31,33-36]. In a randomized study, Carmona et al. [29] reported a significantly higher recurrence rate at the 1-year follow-up in the ablation group than the cystectomy group (31% vs. 11%, p<0.05). However, the overall recurrence rate at the 5-year follow-up became similar between the two groups (37% vs. 22%, p>0.05). In a prospective study, Saito et al. [10] reported no recurrence in any patients in the study population.
In a randomized study, Shaltout et al. [33] reported that the insertion of oxidized regenerated cellulose (Surgicel) inside the cavity of the cyst significantly lowered the recurrence rate in both the ablation group (27.1% to 10.9%) and the cystectomy group (24.4% to 9.1%). An earlier Cochrane review (published in 2008) included the aforementioned two studies [35,36] and concluded that cystectomy showed a significantly lower recurrence rate (odds ratio [OR], 0.41; 95% CI, 0.18–0.93) [4]. In that review, symptom recurrence was also significantly lower in the cystectomy group (dysmenorrhea: relative risk [RR], 0.15; 95% CI, 0.06–0.38; dyspareunia: RR, 0.08; 95% CI, 0.01–0.51; non-menstrual pelvic pain: RR, 0.10; 95% CI, 0.02–0.56). These seven studies clearly show that the recurrence rate tends to be higher after ablation than after cystectomy.
3. Pregnancy rate
Five studies reported the pregnancy rate after ablation versus cystectomy [32,34-37]. In a randomized study by Beretta et al. [35], the 2-year cumulative pregnancy rate was significantly lower in the ablation group than in the cystectomy group (23.5% vs. 66.7%, p<0.05). In a randomized study by Alborzi et al. [36], the 1-year cumulative pregnancy rate was also significantly lower in the ablation group than in the cystectomy group (23.3% vs. 59.4%, p<0.05).
However, in a subsequent randomized study by Alborzi et al. [37], the pregnancy rate after superovulation was similar between the ablation and cystectomy groups (30% vs. 35.7%). A multicenter case-control study by Mircea et al. [32] showed that the probability of spontaneous pregnancy at 24 and 36 months was similar between the ablation and cystectomy groups (61.3% and 84.4% vs. 69.3% and 78.3%, respectively). In a recent retrospective study, Chen et al. [34] also reported a similar spontaneous pregnancy rate between the ablation and cystectomy groups (73% during 32 months vs. 71% during 30 months).
An earlier Cochrane review (published in 2008) included the aforementioned three studies [35-37] and concluded that cystectomy showed a significantly higher pregnancy rate (OR, 5.21; 95% CI, 2.04–13.29) [4]. However, two subsequent studies reported a similar pregnancy rate between the ablation and cystectomy groups [32,34]. Therefore, more research is needed to demonstrate whether the pregnancy rate is different between ablation and cystectomy.
Some clinicians used a “combination technique” or a “three-stage procedure,” but there are very few comparative studies on these techniques. Therefore, they are briefly presented below. In the combination technique, a large part of the endometrioma wall is first removed by cystectomy, and the remaining 10%–20% of the endometrioma wall close to the hilum is ablated [38]. In a randomized study, the combination technique showed a similar recurrence rate to that achieved using cystectomy (2.0% vs. 5.9% at 6 months postoperatively) [39]. The three-stage procedure refers to drainage of the cyst during laparoscopy, followed by subsequent gonadotropin-releasing hormone agonist treatment, and then ablation of the remains during a second laparoscopy [40]. In a small randomized study, the three-stage procedure showed a smaller serum AMH decrement at 6 months postoperatively (mean, 4.5 to 3.99 ng/mL; p>0.05) compared to cystectomy (mean, 3.9 to 2.9 ng/mL; p<0.05) [41].
Sclerotherapy versus cystectomy
Ovarian cystectomy and ablation are now usually performed via the laparoscopic approach. In contrast, sclerotherapy is a type of non-surgical management of ovarian endometrioma. Sclerotherapy involves performing direct percutaneous puncture of ovarian endometrioma to remove the internal fluid, inserting a sclerosing agent such as ethanol into the cyst cavity, and removing it after a certain period of time (“washing” method). Noma and Yoshida [42] reported a higher recurrence rate after ethanol washing for <10 minutes than after ≥10 minutes (62.5% vs. 9.1%, p<0.05).
Some groups used retention of ethanol, wherein the ethanol is left in situ. In a retrospective study of recurrent endometrioma cases, the washing method for 0–10 minutes showed a non-significantly higher recurrence rate (during 1 year) than the retention method (32.1% vs. 13.3%, p>0.05) [43]. Another retrospective study of recurrent endometrioma cases showed that the washing method (for 10 minutes) led to a significantly lower cure rate (during 1 year) than the retention method (82% vs. 96%) [44].
However, a recent retrospective study of patients with recurrent or bilateral endometrioma found similar 1-year recurrence rates between the washing (for 10 minutes) and retention methods (48.1% versus vs. 37.5%) [45]. In that report, live birth rates (spontaneous or artificial conception) were also similar (40% vs. 46.2%). In another recent retrospective study, the washing method (for 1–3 minutes) showed a smaller AMH decrement at 6 months postoperatively than the retention method (–2.7% vs. –23.6%, p<0.05) [46]. In that report, the overall pregnancy rates (up to 9 years) were similar (47.2% vs. 54.5%). Thus, it remains unclear whether the washing method has a higher recurrence rate than the retention method in sclerotherapy of ovarian endometrioma. More research is needed to demonstrate whether the washing method results in a smaller serum AMH decrement than the retention method.
Direct puncture can be performed using a long aspiration needle (16–17 gauge) or a flexible catheter (i.e., catheter-directed sclerotherapy). In a prospective study (14 women with primary or recurrent endometrioma), catheter-directed sclerotherapy decreased endometrioma size (from 5.8 cm to 1.1 cm), and no recurrence of endometrioma was noted during a mean follow-up of 1 year [47]. The mean preoperative and 6-month postoperative serum AMH levels were similar (from 4.29 to 4.36 ng/mL, p>0.05). Simple aspiration alone is usually not recommended because it has a very high recurrence rate (83%–91.5%) [48,49]. However, Zhu et al. [49] reported that repetitive aspiration tended to decrease the recurrence rate, which was 5.4% after the sixth aspiration.
Table 3 lists eight studies [42,50-56] that compared sclerotherapy versus cystectomy for ovarian endometrioma or sclerotherapy versus no intervention in terms of the serum AMH decrement, recurrence of endometrioma, and the pregnancy rate. Five studies included women undergoing in vitro fertilization and embryo transfer (IVF-ET), and the primary endpoint of those studies was clinical pregnancy rate (or live birth rate) [50-54]. In a study by Alborzi et al. [54], sclerotherapy was performed at the time of ovum pickup, and thereafter patients were followed for clinical pregnancy by IVF-ET or recurrence.

Comparative studies of serum AMH decrement, recurrence of endometrioma, and the pregnancy rate after sclerotherapy versus cystectomy for ovarian endometrioma or versus no intervention
1. Serum AMH decrement
Only two studies described the serum AMH decrement at 6 months postoperatively after sclerotherapy versus cystectomy [55,56]. In a study by Garcia-Tejedor et al. [55], preoperative serum AMH levels were similar between sclerotherapy versus cystectomy (2.20 vs. 1.09 ng/mL), and the 6-month postoperative serum AMH levels were also similar between the two groups (2.02 vs. 1.35 ng/mL). In a study by Koo et al. [56], a serum AMH decrement at 6 months postoperatively was not observed in the sclerotherapy group (2.3 to 2.6 ng/mL, p>0.05), but a significant serum AMH decrement was found in the cystectomy group (3.0 to 1.6 ng/mL, p<0.05). Thus, it remains inconclusive whether sclerotherapy is better than cystectomy in terms of ovarian reserve.
2. Recurrence rate
Four studies described the recurrence rate of endometrioma after sclerotherapy versus cystectomy [42,54-56]. Three studies reported a similar recurrence rate between sclerotherapy and cystectomy, but only one study by Alborzi et al. [54] reported a significantly higher recurrence rate in the sclerotherapy group than in the cystectomy group (34.1% vs. 14.0%, p<0.05). Alborzi et al. [54] explained that the unusually higher recurrence rate in the sclerotherapy group could be attributed to the longer follow‐up period in their study. Nonetheless, the majority of currently available reports show a similar recurrence rate when comparing sclerotherapy versus cystectomy.
3. Pregnancy rate
Two studies described a similar spontaneous pregnancy rate between sclerotherapy and cystectomy [42,55]. Five studies described pregnancy rates via IVF-ET, but the participants in the two comparative arms were quite heterogeneous [50-54]. Yazbeck et al. [50] compared IVF-ET outcomes between sclerotherapy and cystectomy groups in a prospective study of patients with recurrent endometrioma. The ongoing pregnancy rates after one IVF cycle (48.3% vs. 19.2%, p=0.04) and after three IVF cycles (55.2% vs. 26.9%, p=0.03) were significantly higher in the sclerotherapy group.
Aflatoonian et al. [51] compared IVF-ET outcomes between the sclerotherapy group for patients with recurrent endometrioma and currently recurring endometrioma (i.e., no intervention) in a randomized study, and the pregnancy rates after one IVF cycle were similar (27.8% vs. 15%, p>0.05). In a retrospective study, Lee et al. [52] compared IVF-ET outcomes between patients who underwent sclerotherapy for recurrent endometrioma, currently recurring endometrioma group (after previous cystectomy), and current endometrioma groups . The pregnancy rates after one IVF cycle were similar (44.4% vs. 37.1% vs. 41.1%).
Miquel et al. [53] compared IVF-ET outcomes between a sclerotherapy group and a current endometrioma group in a retrospective study, and the live birth rate after multiple IVF cycles was significantly higher in the sclerotherapy group (31.3% vs. 14.5%, p<0.05). In a prospective study, Alborzi et al. [54] compared IVF-ET outcomes between the sclerotherapy group and the cystectomy group, and the live birth rates after one IVF cycle were similar (29.5% vs. 38.6%). In that study, sclerotherapy was performed at the time of oocyte pickup; thus, the sclerotherapy group could be interpreted as currently having endometrioma, at least at the time of oocyte pickup.
The five studies regarding the pregnancy rate via IVF-ET in women with endometrioma can be summarized as follows. (1) For recurrent endometrioma, sclerotherapy may be more beneficial than cystectomy (based on one study) [50]. (2) For recurrent endometrioma, sclerotherapy may not be more beneficial than no sclerotherapy (based on two studies) [51,52]. (3) For endometrioma, sclerotherapy may be more beneficial than no sclerotherapy in terms of the cumulative live birth rate (based on one study) [53].
Thus, there is no concrete evidence that sclerotherapy helps to improve the IVF pregnancy rate (when compared to cystectomy or no sclerotherapy). However, the spontaneous pregnancy rate was similar between sclerotherapy and cystectomy. In women with recurrent endometrioma after surgery, cystectomy is a very difficult option to choose because of a diminished ovarian reserve. As an alternative, sclerotherapy can be a good option for recurrent endometrioma, but the sclerotherapy-related decrement of serum AMH and reproductive outcomes should be further evaluated.
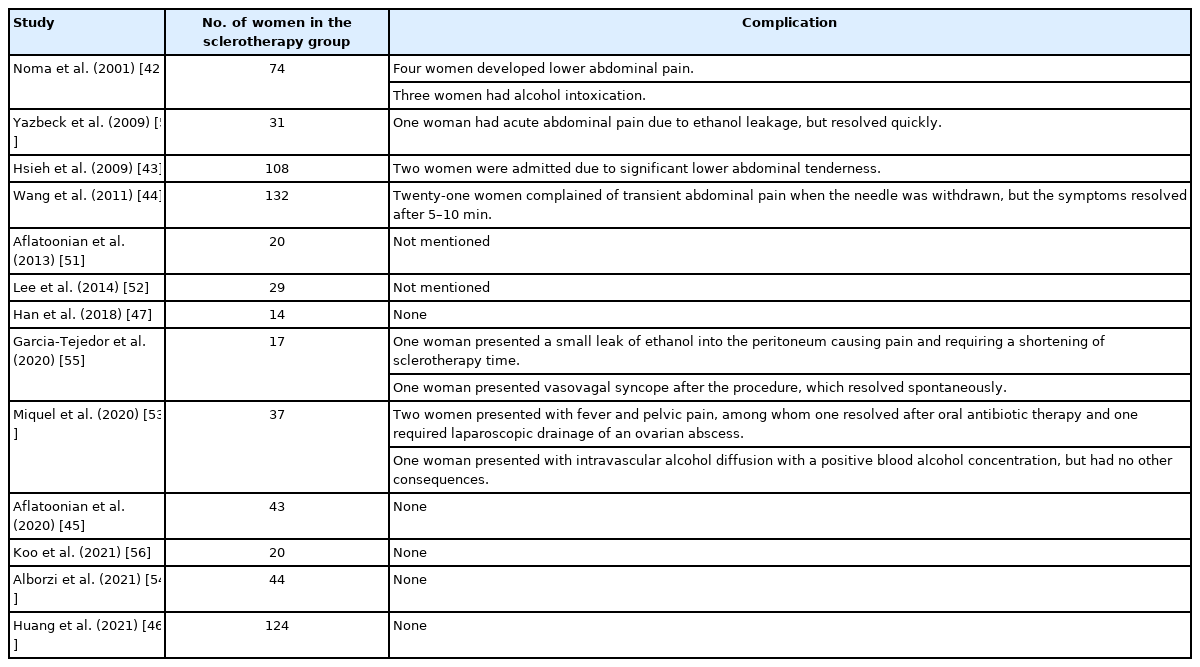
Sclerotherapy can induce abdominal pain (due to ethanol leakage into the peritoneal cavity), intraperitoneal hemorrhage, peritonitis, ovarian abscess, and systemic absorption-related acute alcohol intoxication. Table 4 lists the aforementioned comparative or non-comparative studies and presents the complications of sclerotherapy in detail. The overall crude complication rate was 5.2% (36/693).
Conclusions
The findings of this review can be summarized as follows. First, when cystectomy of ovarian endometrioma is performed, it remains unclear whether suturing or the use of hemostatic agents as a method of hemostasis results in a smaller serum AMH decrement than bipolar coagulation. Second, both ablation and cystectomy have a negative impact on ovarian reserve, but ablation results in a smaller serum AMH decrement than cystectomy. Thus, ablation can be recommended in terms of ovarian reserve. However, ablation tends to result in a higher recurrence rate than cystectomy. In the past, ablation has been reported to be disadvantageous in terms of the pregnancy rate in comparison to cystectomy; however, several recent reports have presented similar pregnancy rates between the two groups. Therefore, more studies are needed to demonstrate whether the pregnancy rate is different between ablation and cystectomy.
Third, when sclerotherapy of ovarian endometrioma is performed, it remains unclear whether the washing method has a higher recurrence rate than the retention method. In addition, more research is needed to show whether the washing method results in a smaller serum AMH decrement than the retention method. Last, it remains inconclusive whether sclerotherapy is better than cystectomy in terms of ovarian reserve. The recurrence rate appears to be similar after sclerotherapy and cystectomy. There is no concrete evidence that sclerotherapy helps to improve the IVF pregnancy rate when compared to cystectomy or no sclerotherapy. In the author's opinion, sclerotherapy should be applied carefully only to recurrent endometriomas when it would be difficult to perform cystectomy or ablation.
Notes
Conflict of interest
Byung Chul Jee has been the editor-in-chief of Clinical and Experimental Reproductive Medicine since 2018; however, he was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflict of interest relevant to this article was reported.