 |
 |
- Search
| Clin Exp Reprod Med > Volume 48(4); 2021 > Article |
|
Abstract
Objective
The impact of early mechanical removal of cumulus cells on fertilization and embryonic development is not yet precisely known. This study aimed to investigate the effects of early and late cumulus cell removal on fertilization, polyspermy, embryonic development potential, blastocyst development, and clinical outcomes.
Methods
A prospective study was conducted of patients who underwent in vitro fertilization between September 2019 and October 2020. Sibling oocytes were randomly allocated after insemination to early cumulus cell removal at 6 hours (group I) and late cumulus cell removal at 16–18 hours (group II). If total fertilization failure (TFF) was determined to have occurred at early cumulus cell removal, rescue intracytoplasmic sperm injection (ICSI) was performed. Fertilization, embryonic development, and pregnancy outcomes were compared.
Cumulus cells play a significant role in supporting oocytes and fertilization. In natural conception, cumulus-enclosed oocyte and spermatozoa meet together at the ampulla of the uterine tube. Cumulus cells enhance capacitation, sperm binding and penetration of the zona pellucida, passage through the perivitelline space, binding and fusion to the oolemma, activation of the cortical reaction, and formation of the male pronucleus. They can also provide nutrients, hormones, and glycosaminoglycans, which are essential for the growth and development of embryos. Removal of the cumulus cells at this moment can markedly decrease fertilization. In conventional in vitro fertilization (IVF), co-incubation of the cumulus-oocyte complex and sperm for 16–18 hours can imitate natural conditions, but prolonged exposure time may generate high levels of reactive oxygen species produced by cumulus cells and excess spermatozoa, resulting in impaired embryo development and vitality [1,2]. Rescue intracytoplasmic sperm injection (ICSI) of unfertilized oocytes in such a situation has shown disappointing results [3]. Nevertheless, it is very important to determine how to improve treatment outcomes. In current practice, short co-incubation of the cumulus-oocyte complex with sperm and early cumulus cell removal (6 hours) is challenging [4-7].
The impact of early mechanical denudation of cumulus cells on fertilization and embryonic development is not yet precisely known. Early cumulus cell removal has been claimed to yield better embryo quality [4,5], but some studies showed comparable results [6,7] and others revealed conflicting outcomes [8]. However, an additional benefit of early cumulus cell removal is early recognition of fertilization based on the presence of a second polar body, and rescue ICSI can be conducted in cases of failed or low fertilization with promising outcomes [7-9]. However, there is still no consensus regarding early cumulus cell removal. This study aimed to investigate the effects of early cumulus cell removal compared to late cumulus cell removal on fertilization, polyspermy, embryonic development potential, blastocyst development, and clinical outcomes.
This prospective randomized clinical study was carried out at Buddhachinaraj Hospital between September 2019 and October 2020. The study was registered with the Thai Clinical Trial Registry (TCTR 20190817001) and approved by the Research Ethics Committee of Buddhachinaraj Hospital Medical School (IRB 066/62). Patients who attended the infertility clinic and had indications for assisted reproduction were invited to join the study and provided written informed consent after enrollment. The inclusion criteria were women undergoing their first IVF treatment cycle aged 20–38 years old, who had at least 6 retrieved oocytes and whose partners had normal semen parameters. The etiologies of infertility included ovulatory dysfunction, tubal disease, unexplained infertility, and endometriosis. The oocytes were randomly allocated into two groups: early cumulus cell removal (group I), in which cumulus cells were removed 6 hours after insemination, and late cumulus cell removal (group II), in which cumulus cells were removed 16–18 hours after insemination. Rescue ICSI was conducted when total fertilization failure (TFF) was expected to occur in the early cumulus cell removal group 6 hours after insemination. Embryo transfers were selected randomly in the two groups in terms of whether the transferred embryos originated from the early or late cumulus cell removal group.
Controlled ovarian hyperstimulation was started with a gonadotropin-releasing hormone agonist for down regulation in the mid-luteal phase of the previous cycle. Follicle-stimulating hormone and/or human menopausal gonadotropin in individually adjusted doses were administered after pituitary desensitization. If at least three follicles were ≥18 mm, 5,000–10,000 IU of human chorionic gonadotropin was injected to induce ovulation. The oocytes were then retrieved with a single-lumen needle under transvaginal ultrasound guidance 36–38 hours later.
Semen samples were gathered by masturbation in the morning on the same day of oocyte collection following 3–5 days of sexual abstinence. Sperm concentration, motility, and morphology were examined under a light microscope based on the World Health Organization criteria (fifth edition, 2010). Gradient centrifugation was used for sperm preparation. Active motile spermatozoa were harvested for insemination, with 20,000–50,000 spermatozoa contained in 50 μL of insemination medium.
The oocytes were transferred to a new sperm-free medium after 4 hours of co-incubation. In group I, the cumulus cells were mechanically removed at 6 hours post-insemination using a denuding pipette (Flexipet; Cook, Brisbane, Australia) with an inner diameter of 140 μm under an inverted microscope. Fertilization was considered to have occurred when a second polar body was present, and TTF was deemed to have occurred when in the absence of a second polar body in any of the mature oocytes. Rescue ICSI was done if none of the oocytes showed early fertilization after insemination for 6 hours. In group II, the cumulus cells were removed at 16–18 hours after insemination.
The developmental competence of zygotes was evaluated after 96–120 hours. Normal fertilization was defined as the presence of 2 pronuclei and polyspermy as the presence of ≥3 pronuclei. Embryos were placed in cleavage medium during days 1–3 after fertilization, followed by blastocyst medium during days 4–5. Embryo morphology was assessed on day 5. The blastocysts were assigned a score based on the Gardner system, with high-quality blastocysts having scores of ≥4 BB [10]. The surplus high-scoring blastocysts were cryopreserved for future transfers.
Embryo transfer took place on day 5 under ultrasound guidance. The number of embryos was limited to one blastocyst to reduce the risk of multiple pregnancies. Progesterone was started on day 3 after oocyte retrieval for luteal support. The implantation rate was defined as the number of gestational sacs divided by the number of embryos that were transferred. The fetal heartbeat demonstrated by ultrasonography was considered to be clinical pregnancy at 5 weeks after embryo transfer. Information was gathered on ongoing pregnancy at 20 weeks and the birth outcomes. Premature delivery was defined as a baby born before 37 weeks.
Continuous data were compared using the Student t-test, and proportional data were compared with the chi-square test and the Fisher exact test. A p-value <0.05 was considered to indicate statistical significance. The analyses were performed using SPSS ver. 13.0 (SPSS Inc., Chicago, IL, USA). A sample size calculation found that at least 240 oocytes in each arm would be satisfactory for a 10% difference in maturation rates between oocytes in the two groups, given a type I error of 5% (two-tailed) and a type II error of 20%.
A total of 85 patients were eligible for the study. Seven patients were excluded due to TTF, and rescue ICSI was performed (Figure 1). The remaining 78 patients were 32.8±2.6 years old. Thirty-three patients (42.3%) had tubal factor fertility, 8 (10.2%) had endometriosis, 10 (12.8%) had polycystic ovary syndrome, and 27 (34.7%) had unexplained infertility (Table 1). In total, 912 oocytes were randomly allocated to early cumulus cell removal (group I, 458 oocytes) and late cumulus cell removal (group II, 454 oocytes). The details of fertilization and embryo development are summarized in Table 2. No significant differences were observed in fertilization, cleavage rate, and embryo quality. Polyspermy was higher in group I than in group II (9.0% vs. 5.9%, respectively), but the difference did not reach statistical significance.
Embryo transfer was conducted in 78 patients, of whom 40 patients obtained embryos from group I and 38 patients from group II. There were no significant differences between the two groups in terms of age, and the number of oocytes. Implantation rates, clinical pregnancy, ongoing pregnancy, and live birth rate were not significantly different between the patients with embryos transferred from the early and late cumulus cell removal groups (Table 3). No twin pregnancies occurred in this study.
Notably, seven patients in the early cumulus cell removal group had TTF, and rescue ICSI was carried out. Nineteen of 24 oocytes (79.2 %) had normal fertilization without detected polyspermy and three embryos were transferred in three cycles leading to one single pregnancy with an uneventful course of gestation and delivery of a normal newborn (Table 4). However, in the late cumulus cell removal group, four patients had simultaneous fertilization in the sibling oocytes of the same patients, the embryos were transferred with a successful singleton, and there was a normal course of pregnancy with breech presentation, followed by delivery of a normal infant by cesarean section (Table 5).
There are inconsistent reports on the outcomes of early cumulus cell removal. Some studies have shown that early cumulus cell removal has fertilization and clinical pregnancy rates similar to late removal at 20 hours [6,7]. Another study demonstrated that early cumulus removal after 4 hours of insemination resulted in low numbers of available embryos [8]. However, a meta-analysis [11] showed that early cumulus cell removal was associated with a significant increase in the implantation rate and clinical pregnancy rate. The ability to draw a clear conclusion may be hindered by differences in study designs and populations. In the present study, sibling oocytes were randomly allocated into two groups to minimize the confounding between patients, and the findings indicated that early cumulus cell removal had no significant difference in fertilization, polyspermy, cleavage of embryos, clinical pregnancy, and ongoing pregnancy, comparable with the findings of previous studies [4,5,12-16].
Conventionally, cumulus cells are recognized as essential for oocyte development and the natural fertilization process, but become less important after ICSI because embryos can develop normally without cumulus cells. This fact implies that the early removal of cumulus cells may not affect embryonic development. Moreover, Nagy et al. [17] found that oocytes were fertilized 2-4 hours after exposure to spermatozoa and the second polar body was extruded by approximately 90% into perivitelline space by 6 hours [6]. If this event is observed during early cumulus cell removal, rescue ICSI can be performed, resulting in higher fertilization rates and optimal embryos compared with rescue ICSI after late cumulus cell removal (20 hours) [6]. Interestingly, the time-course of fertilization in early rescue ICSI has a similar pattern to those oocytes that undergo ICSI at the normal time of fertilization, allowing the embryos to be obtained in synchronized development with the endometrium [6]. In this study, cumulus cells were dissected at 6 hours and rescue ICSI was conducted in seven cases of TFF, which obtained a satisfactory fertilization rate (79.2%); thus, meant ICSI after early cumulus cell removal (6 hours) could rescue most of the unfertilized oocytes. Therefore, early cumulus cell removal in conjunction with rescue ICSI provides an additional benefit by alleviating cycle cancellation in patients with TFF. Nevertheless, it is possible that injecting fertilized eggs can delay extrusion of the second polar body (3.2%) [9]. To avoid such an event, rescue ICSI can be postponed to 9 hours after insemination [12]. Remarkably, polyspermy after rescue ICSI was not found even when performed at 6 hours in our study. Despite the potential advantage of early cumulus cell removal and rescue ICSI, the demand of embryologists to cover the additional work during the extra period must be considered [6,11].
This study found a trend toward a higher polyspermy rate, albeit without statistical significance, in early cumulus cell removal (9.0%) than in late removal (5.9%), which aligns with previous reports [7,15]. It is unclear whether early cumulus cell removal can affect polyspermy. Cumulus cells are tougher and more difficult to remove at the earlier time point than at the later time point. However, at an early period after insemination, the oocytes are also more vulnerable due to their active spindles and microtubules [18,19]. Thus, they may have more susceptible to damage from the additional mechanical force created by the denuding pipette during cumulus cell removal [16]. Additionally, repeated mechanical stress can also have adverse effects on the integrity of the zona pellucida and may reduce the protective mechanism against polyspermic fertilization [16]. Of particular note, unstable culture conditions or an excessive number of sperm in the culture medium can also affect abnormal fertilization through different pathways [12]. These possible mechanisms may contribute to an increase in polyspermy. However, a meta-analysis showed that the use of a denuding pipette during cumulus cell removal was not harmful to the clinical pregnancy or implantation rate [11].
Our results showed that early cumulus cell removal had comparable obstetric and prenatal outcomes with late cumulus cell removal, in agreement with Liu et al. [14]. However, recent studies [11,13] reported higher rates of premature delivery, twins, and low-birth-weight newborns in patients who underwent early cumulus cell removal. They proposed that the process during removal of cumulus cell at the early time point could possibly alter spindle integrity and impair cell division more than late removal, which might give rise to twins or poor fetal growth [20]. In addition, inappropriate mechanical forces during cumulus cell removal can cause epigenetic changes associated with low birth weight [21,22]. Further studies should be conducted to elucidate this interesting issue.
The present study showed that early cumulus cell removal at 6 hours after insemination had no significant difference in fertilization, polyspermy, embryo development, obstetric and perinatal outcomes. Early cumulus cell removal combined with early rescue ICSI may have the potential to help couples with TTF.
Figure 1.
Consolidated standards of reporting trials (CONSORT) flowchart. ICSI, intracytoplasmic sperm injection.
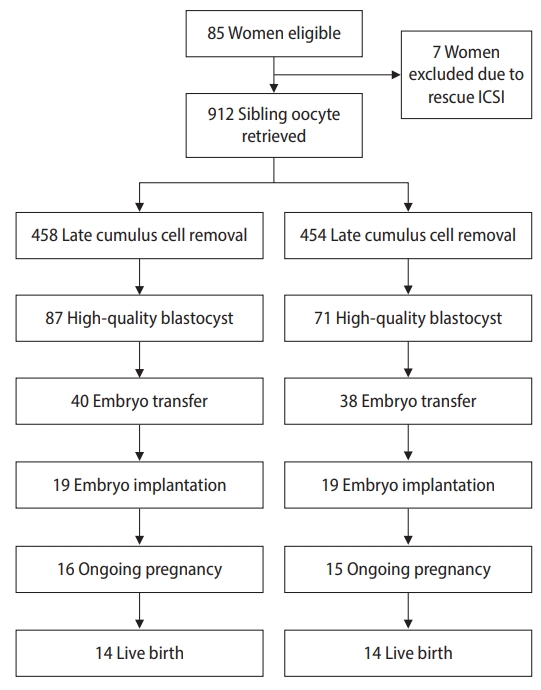
Table 1.
Baseline characteristics of the 78 patients who participated in the study
| Characteristics | Value |
|---|---|
| Age (yr) | 30.4±3.7 |
| Body mass index (kg/m2) | 25.5±2.6 |
| Etiology of infertility | |
| Tubal factor | 33 (42.3) |
| Endometriosis | 8 (10.2) |
| PCOS | 10 (12.8) |
| Unexplained infertility | 27 (34.7) |
Table 2.
Outcomes of oocyte and embryo development using early and late cumulus cell removal
| Variable | Early removal (group I) | Late removal (group II) | p-valuea) |
|---|---|---|---|
| Mature oocytes | 458 | 454 | - |
| Two pronuclei | 375 (81.9) | 373 (82.2) | 0.913 |
| Polyspermy | 41 (9.0) | 27 (5.9) | 0.111 |
| Cleavage | 320 (85.3) | 302 (81.0) | 0.112 |
| Blastocyst formation | 178 (55.6) | 156 (51.7) | 0.323 |
| High quality blastocyst | 87 (27.1) | 71 (23.5) | 0.294 |
Table 3.
Pregnancy outcomes according to early and late cumulus cell removal
| Variable | Early removal (group I) | Late removal (group II) | p-value |
|---|---|---|---|
| No. of patients | 40 | 38 | - |
| Mean age (yr) | 32.2±2.7 | 33.3±2.5 | 0.066a) |
| No. of embryos transferred | 1 | 1 | - |
| Implantation rate | 19/40 (47.5) | 19/38 (50.0) | 0.995b) |
| Clinical pregnancy rate | 18/40 (45.0) | 17/38 (44.7) | 0.838b) |
| Ongoing pregnancy rate | 16/40 (40.0) | 15/38 (39.5) | 0.854b) |
| Live birth rate | 14/40 (35.0) | 14/38 (36.8) | 0.947b) |
| Premature delivery rate | 2/16 (12.5) | 1/15 (6.7) | 0.999c) |
| Birth weight (g) | 2,930±266 | 3,040±250 | 0.059a) |
Table 4.
Details of embryonic competence and clinical outcomes in seven patients who had rescue ICSI
| Patient | Age (yr) | Oocyte | Fertilization | Cleavage | Blastocyst | Implantation | Clinical pregnancy | Birth |
|---|---|---|---|---|---|---|---|---|
| 1a) | 28 | 4 | 3 | 3 | 2 | 0 | 0 | 0 |
| 2a) | 30 | 3 | 3 | 3 | 3 | 1 | 1 | 1 |
| 3b) | 31 | 4 | 4 | 3 | 1 | - | - | - |
| 4b) | 31 | 3 | 2 | 2 | 2 | - | - | - |
| 5b) | 32 | 3 | 2 | 2 | 1 | - | - | - |
| 6a) | 34 | 4 | 3 | 2 | 2 | 0 | 0 | 0 |
| 7b) | 37 | 3 | 2 | 2 | 1 | - | - | - |
Table 5.
Details of embryonic competence and clinical outcomes of late cumulus removal oocytes in four patients who had TFF of sibling oocytes of early cumulus removal
| Patient | Age (yr) | Oocyte | Fertilization | Cleavage | Blastocyst | Implantation | Clinical pregnancy | Birth |
|---|---|---|---|---|---|---|---|---|
| 3 | 31 | 4 | 3 | 3 | 2 | 0 | 0 | 0 |
| 4a) | 31 | 4 | 3 | 3 | 3 | 1 | 1 | 1 |
| 5b) | 32 | 4 | 4 | 2 | 2 | 0 | 0 | 0 |
| 7 | 37 | 3 | 2 | 2 | 1 | 0 | 0 | 0 |
References
1. Bedaiwy MA, Falcone T, Mohamed MS, Aleem AA, Sharma RK, Worley SE, et al. Differential growth of human embryos in vitro: role of reactive oxygen species. Fertil Steril 2004;82:593-600.


2. Enkhmaa D, Kasai T, Hoshi K. Long-time exposure of mouse embryos to the sperm produces high levels of reactive oxygen species in culture medium and relates to poor embryo development. Reprod Domest Anim 2009;44:634-7.


3. Yuzpe AA, Liu Z, Fluker MR. Rescue intracytoplasmic sperm injection (ICSI)-salvaging in vitro fertilization (IVF) cycles after total or near-total fertilization failure. Fertil Steril 2000;73:1115-9.


4. Kattera S, Chen C. Short coincubation of gametes in in vitro fertilization improves implantation and pregnancy rates: a prospective, randomized, controlled study. Fertil Steril 2003;80:1017-21.


5. Dirnfeld M, Shiloh H, Bider D, Harari E, Koifman M, Lahav-Baratz S, et al. A prospective randomized controlled study of the effect of short coincubation of gametes during insemination on zona pellucida thickness. Gynecol Endocrinol 2003;17:397-403.


6. Chen C, Kattera S. Rescue ICSI of oocytes that failed to extrude the second polar body 6 h post-insemination in conventional IVF. Hum Reprod 2003;18:2118-21.


7. Lundqvist M, Johansson U, Lundkvist O, Milton K, Westin C, Simberg N. Reducing the time of co-incubation of gametes in human in-vitro fertilization has no beneficial effects. Reprod Biomed Online 2001;3:21-4.


8. Barraud-Lange V, Sifer C, Pocate K, Ziyyat A, Martin-Pont B, Porcher R, et al. Short gamete co-incubation during in vitro fertilization decreases the fertilization rate and does not improve embryo quality: a prospective auto controlled study. J Assist Reprod Genet 2008;25:305-10.



9. Dai SJ, Qiao YH, Jin HX, Xin ZM, Su YC, Sun YP, et al. Effect of coincubation time of sperm-oocytes on fertilization, embryonic development, and subsequent pregnancy outcome. Syst Biol Reprod Med 2012;58:348-53.


10. Tucker MJ, Liebermann J. Morphological scoring of human embryos and its relevance to blastocyst transfer. In: Patrizio P, Tucker MJ, Guelman V, editors. A color atlas for human assisted reproduction. Philadelphia: Lippincott William & Wilkins; 2003. p. 99-108.
11. Zhang XD, Liu JX, Liu WW, Gao Y, Han W, Xiong S, et al. Time of insemination culture and outcomes of in vitro fertilization: a systematic review and meta-analysis. Hum Reprod Update 2013;19:685-95.


12. Liu J, Zhang X, Yang Y, Zhao J, Hao D, Zhang J, et al. Long-time vs. short-time insemination of sibling eggs. Exp Ther Med 2016;12:3756-60.



13. Guo N, Yang F, Liu Q, Ren X, Zhao H, Li Y, et al. Effects of cumulus cell removal time during in vitro fertilization on embryo quality and pregnancy outcomes: a prospective randomized sibling-oocyte study. Reprod Biol Endocrinol 2016;14:18.



14. Liu J, Chen M, Lin C, Weng X, Meng Z, Tang W. Effect of early cumulus cell removal on the fertilization and clinical outcome in human in vitro fertilization. Adv Reprod Sci 2015;3:50-6.
15. Xue Y, Tong X, Jiang L, Zhu H, Yang L, Zhang S. Effect of cumulus cell removal 4 h post-insemination on fertilization and embryo quality: a prospective randomized sibling-oocyte study. J Assist Reprod Genet 2013;30:1049-53.



16. Xiong S, Han W, Liu JX, Zhang XD, Liu WW, Liu H, et al. Effects of cumulus cells removal after 6 h co-incubation of gametes on the outcomes of human IVF. J Assist Reprod Genet 2011;28:1205-11.



17. Nagy ZP, Liu J, Joris H, Devroey P, Van Steirteghem A. Time-course of oocyte activation, pronucleus formation and cleavage in human oocytes fertilized by intracytoplasmic sperm injection. Hum Reprod 1994;9:1743-8.

18. Payne D, Flaherty SP, Barry MF, Matthews CD. Preliminary observations on polar body extrusion and pronuclear formation in human oocytes using time-lapse video cinematography. Hum Reprod 1997;12:532-41.


19. Wang WH, Day BN, Wu GM. How does polyspermy happen in mammalian oocytes? Microsc Res Tech 2003;61:335-41.


20. Li GP, Bunch TD, White KL, Rickords L, Liu Y, Sessions BR. Denuding and centrifugation of maturing bovine oocytes alters oocyte spindle integrity and the ability of cytoplasm to support parthenogenetic and nuclear transfer embryo development. Mol Reprod Dev 2006;73:446-51.


- TOOLS
-
METRICS

- Related articles in Clin Exp Reprod Med
-
Comparison of the clinical outcomes of day 4 and 5 embryo transfer cycles2013 September;40(3)






