 |
 |
- Search
| Clin Exp Reprod Med > Volume 49(4); 2022 > Article |
|
Abstract
Objective
Oxidative stress is a key player in the development of idiopathic male infertility (IMI), and various antioxidants have been used for the treatment of IMI with inconsistent results. Coenzyme Q10 (CoQ10) is a cofactor and an antioxidant that may improve semen parameters and reduce oxidative stress in patients with idiopathic oligoasthenospermia (OA). Therefore, this study aimed to explore the effect of CoQ10 on semen parameters and antioxidant markers in patients with idiopathic OA.
Methods
Fifty patients with idiopathic OA and 35 fertile controls were enrolled in this prospective controlled study. All participants underwent a comprehensive fertility assessment. All patients received CoQ10 (300 mg/day) orally once daily for 3 months. Semen parameters, seminal CoQ10 levels, reactive oxygen species (ROS) levels, total antioxidant capacity (TAC), superoxide dismutase (SOD), and glutathione peroxidase (GPx) were measured in patients and controls at the start of the study and after 3 months.
Results
Treatment with CoQ10 resulted in increased sperm progressive motility (p<0.05), total motility (p<0.01), seminal TAC (p<0.01), SOD (p<0.05), GPx (p<0.001), and seminal CoQ10 (p<0.001) levels and reduced ROS (p<0.01) in patients as compared to baseline. Sperm concentration and motility were also significantly correlated with antioxidant measures and seminal CoQ10 levels (r=0.38–0.57).
Infertility refers to the inability of a couple to conceive after 12 months of regular unprotected intercourse [1]. Infertility affects approximately 10% to 15% of couples worldwide [2,3]. Male factors contribute to approximately half of these cases [4]. Oligoasthenospermia (OA) is diagnosed when a low sperm concentration (<15 million/mL) and motility (progressive motility <32%, total motility <40%) are identified according to World Health Organization (WHO) guidelines [5]. Several factors have been linked to OA, including autoimmunity, cryptorchidism, varicocele, systemic diseases, endocrine diseases, genital trauma, genital infection, medications, and toxins [6]. However, in approximately 25% of cases, the underlying causes of OA cannot be identified; these cases are designated as idiopathic male infertility (IMI) [7]. Several mechanisms have been suggested for IMI, including genetic and epigenetic factors, oxidative stress (OS), and sperm DNA fragmentation (SDF).
OS is the result of an imbalance between pro-oxidants and antioxidant defense mechanisms, which results in a state of redox paradox [8]. Reactive oxygen species (ROS) are essential for certain physiological functions required for male reproduction. However, high levels of ROS are detrimental to sperm, as sperm have limited intrinsic antioxidant capacity. OS could be caused by endogenous factors such as metabolic processes, immature sperm, and leukocytes, as well as exogenous factors such as infection, varicocele, smoking, alcohol, obesity, malignancy, radiotherapy, chemotherapy, environmental toxins, and systemic disease. The seminal fluid contains a set of antioxidants that protect spermatozoa from oxidative damage. These antioxidants include enzymatic antioxidants, such as catalase (CAT), glutathione peroxidase (GPx), and superoxide dismutase (SOD) [9], as well as non-enzymatic components including vitamins A, C, and E, coenzyme Q10 (CoQ10), pyruvate, L-carnitine, glutathione, taurine, urate, zinc, and selenium [10]. Excessive production of ROS and OS could lead to sperm membrane lipid peroxidation, increased sperm membrane permeability, reduced sperm motility, reduced fertilization, SDF, and poor pregnancy and assisted reproductive techniques (ART) outcomes [11]. These adverse effects may reduce male fertility potential and increase the risk of poor pregnancy outcomes. Increased SDF may also result in abnormal sperm function, reduced male fertility potential, and reduced pregnancy and ART success rates [12]. Recent studies have also linked OS and SDF to the development of autosomal dominant genetic diseases, childhood malignancies, birth defects, and neurological diseases in newborns [13].
Previous studies have shown that oral antioxidants may enhance semen parameters and seminal antioxidant status [14,15]. However, there is a lack of consensus on the type of antioxidant, dosing, length of therapy, and target group [16]. Several studies have tried different doses of the same antioxidant or compared two or more antioxidants and different treatment durations, which illustrates this inconsistency. In addition, the excessive use of antioxidants and high doses may reduce ROS and may shift the redox balance into a reductive status, leading to a state of reductive stress [17]. CoQ10 is a cofactor, and its reduced form (ubiquinol) has antioxidant and mitochondrial bioenergetic properties [18]. It transfers electrons in the mitochondria from complex I/II to complex III. CoQ10 is widely distributed in human tissues, and its main dietary sources include oily fish, meat, and whole grains. Reduced CoQ10 levels are associated with cardiovascular diseases, diabetes mellitus, and cancer, and seminal CoQ10 levels are also reduced in infertile men, which correlates with sperm concentration and motility [19]. We and others have also shown that CoQ10 treatment improves semen measures and seminal antioxidant status in patients with infertility [20,21]. Furthermore, our recent meta-analysis of three randomized controlled trials (RCTs) also confirmed beneficial effects for CoQ10 on sperm motility with a longer duration of treatment of 6 months [22]. A single study, however, demonstrated a lack of improvement in semen measures following CoQ10 treatment [23]. While the aforementioned studies explored the impact of various antioxidants on semen measures in patients with infertility and reported beneficial effects, studies on the effect of CoQ10 on seminal OS levels in patients with idiopathic OA are scarce. Furthermore, inconsistencies regarding the optimal dose, duration of treatment, and the target group for CoQ10 therapy exist. In addition, managing the challenging group of men with IMI requires the development of an optimal treatment protocol for oral antioxidant therapy including CoQ10. Therefore, this study aimed to explore the effect of CoQ10 therapy for 3 months on improving semen measures and seminal antioxidant status in men with idiopathic OA and fertile controls. To our knowledge, the study is the first to explore the impact of CoQ10 (300 mg/day) on seminal antioxidant markers in infertile patients with idiopathic OA.
Ethical approval was received from the University of Sumer, Iraq (EC/2018/8876) and informed consent was obtained before enrollment.
In this prospective controlled study, 50 infertile men with idiopathic OA (mean age, 27.28±8.47 years; mean duration of infertility, 5.62±3.49 years) and 35 fertile healthy control subjects (mean age, 29.61±9.33 years) were included. Five patients did not complete the study and therefore were excluded. The patients were selected from the Fertility Clinic, Hillah, Iraq from May to September 2018. A special questionnaire was designed to collect data. All patients underwent a detailed fertility assessment by a fertility specialist to exclude known causes of OA. For all participants, semen analysis (WHO guidelines), seminal antioxidant measures (total antioxidant capacity [TAC], SOD, GPx, and ROS), and seminal fluid CoQ10 levels were assessed at the start of the study and after 3 months. Patients received CoQ10 (300 mg/day) orally in a single dose for 3 months and their measures were compared between baseline and after CoQ10 therapy. The dose of CoQ10 was similar to the dose used in previous studies [24]. The sample size, which was calculated using 80% power and a 5% significance level, was 34 for each group. The test is based on superiority and we used a previously published method for sample size calculation [25].
Patients had a history of infertility of at least 1 year despite regular unprotected intercourse. OA was diagnosed when abnormal sperm concentration and motility according to the fifth-edition WHO criteria were detected in semen analysis [26]. Patients who had varicocele, genital tract infection, scrotal trauma, scrotal surgery, undescended testis, azoospermia, systemic illness, smoking, alcohol use, female factor infertility, and recent antioxidant intake in the last 6 months were excluded from the study. Fertile controls were required to have fathered a child in the last 2 years and have a normal semen analysis.
Semen samples were obtained by masturbation after sexual abstinence for 2–3 days, collected into a special container, held at 37°C for liquefaction, and then semen analysis was performed within 1 hour according to the fifth edition WHO guidelines [26] for all semen parameters and the fourth edition WHO guidelines for sperm morphology depending on the available laboratory facilities [27]. Semen analyses were performed by the same researcher. Two semen samples were assessed at the start of the study and after 3 months, and their average value was used for this study.
Semen samples were centrifugated at 3,000 rpm for 5 minutes, and seminal plasma was stored at –20°C. The TAC level was assessed with the total antioxidant capacity assay kit (#E-BC-K136; Elabscience, Houston, TX, USA). Colorimetric optical absorbance was measured at 520 nm using the standard procedure.
SOD activity was assessed according to the method reported by Magnani et al. [28]. The test principle is based on the competition between pyrogallol autoxidation by O2•− and scavenging by SOD. Colorimetric optical absorbance was measured at 420 nm using the standard procedure.
GPX was assessed with the glutathione peroxidase (GSH-PX) assay kit (#E-BC- K096, Elabscience). Colorimetric optical absorbance was measured at 412 nm using the standard procedure.
ROS was assessed using a manual method reported by Venkatesh et al. [29]. The level of ROS was measured using luminol-dependent chemiluminescence. Reverse-phase high-performance liquid chromatography was utilized to assess seminal CoQ10 levels using an ultraviolet detector at 275 nm according to the method described by Li et al. [30]. Coenzyme Q9 was used as the internal standard.
The statistical analysis was conducted using IBM SPSS ver. 24 (IBM Corp., Armonk, NY, USA). The results were described as mean ±standard deviation. The normality of data was explored using the Kolmogorov-Smirnov test, and a normal distribution was shown (p>0.05). The dependent t-test was used for comparisons of parameters before and after treatment, and the independent t-test was used to compare seminal measures between patients and fertile controls. Correlations between seminal parameters and TAC, SOD, GPx, ROS, and seminal CoQ10 levels were estimated using Pearson correlation coefficients. A p-value below 0.05 was adopted as the level for statistical significance.
Patients' and controls' demographics and semen parameters are shown in Table 1. Sperm concentration and motility in the patient group were significantly lower than in the fertile controls (p<0.001). Following 3 months of CoQ10 therapy, sperm progressive motility (p<0.05) and total motility (p<0.01) in patients improved significantly in comparison to pretreatment levels. Normal sperm morphology was reduced after CoQ10 therapy, but not to a statistically significant extent (p>0.05). Infertile patients demonstrated lower seminal TAC, SOD, GPX, and CoQ10 levels, and higher seminal ROS than fertile controls (p<0.01) (Figure 1). CoQ10 administration in patients also resulted in significantly higher seminal TAC (p<0.01), SOD (p<0.05), GPX (p<0.001), and CoQ10 levels (p<0.001), and lower seminal ROS levels (p<0.01) post-therapy.
We detected significant and direct correlations of sperm concentration and motility with seminal TAC, SOD, GPx, and CoQ10 levels. Additionally, we observed significant and inverse correlations of sperm concentration and motility with ROS. Seminal CoQ10 levels also showed significant correlations with seminal TAC and SOD (Table 2).
The recent decades have witnessed a decline in semen quality among men worldwide. Infertility represents a global health issue, and IMI is challenging both for science and clinical practice. Infertility imposes medical and psychological consequences on infertile couples as well as a financial burden on health system resources. The exact mechanisms underlying semen abnormalities in men with IMI remain unknown. Furthermore, many treatment modalities for this condition have yielded inconsistent results. Patients with IMI represent a significant proportion of infertile couples and the condition has an impact on infertile couples, as well as on the community and health systems due to the need for long, extensive, and costly investigations and treatment. This study showed that treatment with CoQ10 for 3 months improved sperm progressive and total motility in infertile men with idiopathic OA. Our results are in parallel with other studies that demonstrated increments in sperm motility following the administration of oral antioxidants, including vitamin C, vitamin E, CoQ10, L-carnitine, zinc, selenium, and N-acetylcysteine in infertile men [10,31]. However, these studies demonstrated a lack of agreement on standardized antioxidant regimens. Further, some studies used one or two oral antioxidants, while others explored the effects of combined antioxidants with variable doses for each antioxidant. Balercia et al. [20] conducted a RCT on 60 patients with idiopathic asthenospermia who received CoQ10 (200 mg/day) for 6 months. The study detected an increment in progressive motility in the treatment group. In another RCT, infertile patients with idiopathic oligoasthenoteratospermia (OAT) were treated with CoQ10 (300 mg/day) for 26 weeks and demonstrated improvements in semen parameters post-therapy [32]. Furthermore, a study on men with idiopathic OAT reported an increment in all semen measures following CoQ10 (300 mg/day) for 12 months [33]. Another study investigated the effect of CoQ10 (100 mg/day for 6 months) in patients with idiopathic asthenospermia and reported improvements in both sperm motility and normal morphology [34]. The use of different doses of CoQ10 in the aforementioned studies highlights the need for optimization of the dose and treatment protocol of CoQ10 in infertile patients with IMI.
Our results are also in parallel with our other studies that reported improvements in semen parameters in patients with idiopathic OA and OAT who received CoQ10 (200 mg/day) for 3 months [9,21,35]. Further, our recent meta-analysis of three RCTs and two other recent meta-analyses have also confirmed beneficial effects for CoQ10 on semen parameters (mainly sperm motility) and with a long duration (6 months) of treatment [22,36,37]. Another study, however, demonstrated no significant changes to seminal fluid parameters after CoQ10 treatment [23]. The observed improvement in sperm motility in men with idiopathic OA could be the result of reduced ROS and increased antioxidant capacity (TAC, GPx, and SOD), which could be due to the antioxidant characteristics of CoQ10, its key role as a cofactor for the mitochondrial respiratory chain, and its mitochondrial bioenergetics effects. These effects counteract the detrimental effects of OS on sperm membranes, motility, and fertilization. Additionally, recent reports have suggested anti-apoptotic, anti-inflammatory, and gene modulation effects for CoQ10, which could explain the use of CoQ10 for the treatment of other diseases such as ischemic heart diseases, Parkinson’s disease, diabetes mellitus, and malignant tumors in addition to infertility [36,38,39]. We [12,35,40] and others [41-43] have also detected higher SDF in men with IMI, and these levels were ameliorated by CoQ10 therapy. These findings augment the role of CoQ10 as a gene modulator. One possible explanation for the different results of the previous studies is the heterogeneity of the patient groups (OA, OAT, or asthenospermia), different durations of treatment (3–26 weeks), and the lack of a consensus on the optimal dose of CoQ10. Establishing the optimal dose of CoQ10 and duration of treatment could increase the efficiency of the treatment protocol and may help patients avoid exposure to an unnecessarily high dose of CoQ10 or long treatment.
In our study, CoQ10 therapy in patients also resulted in significantly higher seminal TAC, SOD, GPX, and CoQ10 levels, as well as lower seminal ROS levels, post-treatment. Significant and direct correlations between sperm concentration and motility with antioxidant measures and seminal CoQ10 levels were also detected. Studies have reported that it is essential to identify new biomarkers and predictors of pregnancy and ART outcomes for the evaluation of male infertility, such as sperm function tests, OS markers, and SDF. Further, the correlations detected in this study between semen parameters and antioxidant markers could provide a rationale for the use of different antioxidants as a potential treatment for patients with IMI through reducing seminal OS and increasing antioxidant defenses.
Our results are congruent with those of other studies that have shown reduced seminal antioxidant defenses and CoQ10 levels in infertile patients [41,44]. Administration of CoQ10 could increase seminal CoQ10 and may ameliorate seminal OS [20,45]. Safarinejad et al. [24] reported an increment in semen measures and TAC in patients with idiopathic OAT following CoQ10 (200 mg/day) treatment. Correlations between CAT, SOD, and semen parameters were also observed in this study. Our findings are also in parallel with our previous studies, which demonstrated that CoQ10 therapy (200 mg/day) in men with IMI increased TAC, SOD, CAT, GPx, and seminal CoQ10 levels and reduced seminal ROS levels [9,12,21,35,40]. The detection of higher seminal CoQ10 levels in these patients after CoQ10 treatment also suggests that oral administration of CoQ10 could be effective in increasing seminal CoQ10 levels and seminal antioxidant defenses against OS. It is worth mentioning that in our previous studies, we used a lower dose of CoQ10 (200 mg/day), meaning that a direct comparison of the results may not be possible. In these studies, correlations between sperm concentration and motility with antioxidant markers and seminal CoQ10 levels were also observed. In addition, our recent systematic review and another recent systematic review confirmed the beneficial effects of CoQ10 supplementation on improving semen parameters in men with infertility [36,46]. The improvement in seminal antioxidant capacity in patients with idiopathic OA in our study could have been due to higher seminal CoQ10 levels, antioxidant features of CoQ10, its role as a cofactor in the mitochondrial respiratory chain, and its bioenergetic effects that counteract the detrimental effects of OS on sperm and the fertilization process. Furthermore, recent reports have highlighted the impact of CoQ10 on gene modulation as well as inflammatory and apoptotic inhibitory effects [38,39]. Previous studies have used a wide range of CoQ10 doses (100–400 mg/day), but with inconsistent results. Therefore, our study explored the effect of CoQ10 (300 mg/day) on semen parameters and antioxidant markers in men with idiopathic OA. Data available on the effect of this dose in men with idiopathic OA are limited. The improvement in progressive and total sperm motility, TAC, SOD, GPx, CoQ10 levels, as well as the reduction in ROS detected in our study, were greater than those observed in other studies that have used CoQ10 doses of 100 or 200 mg/day [20,21,34,47,48]. This finding could be attributed to the stronger antioxidant effects of CoQ10, its bioenergetic properties, and enhanced antioxidant defenses, as well as the reduction of ROS with a higher dose of CoQ10. In contrast, recent studies have reported reductive stress with high doses of antioxidants [17]. Therefore, optimizing the dose of antioxidants, including CoQ10, is essential for the effective management of infertile men with IMI. It is worth mentioning that comparing our results with previous studies is challenging due to variability in study design, patient groups, and therapeutic protocols. Some of the studies mentioned above have shown beneficial effects for CoQ10 with a duration ranging from 3 months to 26 weeks, and the fact that more effective results were obtained with a longer duration could be attributed to longer antioxidant effects and the duration of the spermatogenesis cycle of 72 days.
Our study was limited by the small number of control group participants due to consent, and we did not assess dietary CoQ10 intake, although a dietary assessment could be limited by the complexity of dietary intake and recall bias. We did not assess the pregnancy rate because the follow-up was limited by time and cost constraints. Another limitation is the selection of participants from a single area, so our findings may not be generalizable to all regions, and further large and multicenter studies are warranted.
CoQ10 therapy (300 mg/day for 3 months) in patients with idiopathic OA could improve sperm progressive motility, total motility, and seminal antioxidant markers. Therefore, CoQ10 could be a promising treatment for patients with IMI and may enhance their fertility and pregnancy outcomes.
Acknowledgments
The author is very grateful to all the participants of the study for their great help in completing this research.
Figure 1.
(A) Seminal plasma total antioxidant capacity (TAC), (B) superoxide dismutase (SOD), (C) glutathione peroxidase (GPx), (D) reactive oxygen species (ROS), and (E) coenzyme Q10 (CoQ10) levels in controls and infertile patients pre-and post-coenzyme Q10 treatment. Significant difference from patients’ baseline: a)p<0.05, b)p<0.01, c)p<0.001; Significant difference from control: d)p<0.05, e)p<0.01, f)p<0.001.
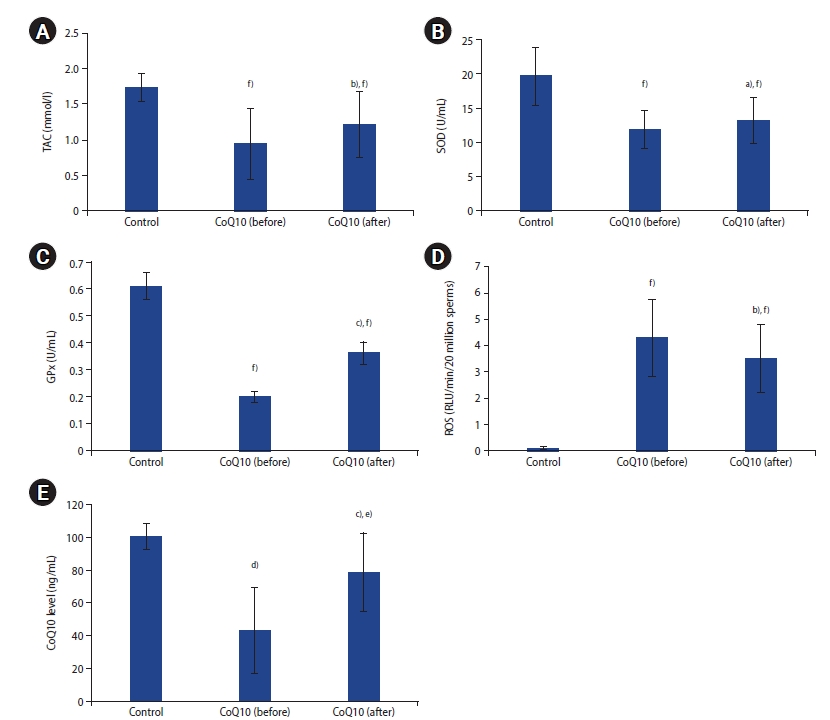
Table 1.
Patients’ and controls’ characteristics and semen parameters
| Semen parameter | Fertile control (n=35) | Patient before CoQ10 (n=50) | Patient after CoQ10 (n=50) |
|---|---|---|---|
| Age (yr) | 29.61±9.33 | 27.28±8.47 | - |
| Infertility duration (yr) | - | 5.62 ±3.49 | - |
| Volume (mL) | 2.75±0.67 | 2.72±0.85 | 2.92±0.78 |
| Concentration (million/mL) | 46.58±24.5 | 8.62±4.51c) | 10.3±5.22c) |
| Progressive motility (%) | 43.5±9.05 | 20.37±7.86c) | 24.6±11.42a),c) |
| Total motility (%) | 60.4±11.26 | 27.4±9.44c) | 33.8±12.61b),c) |
| Normal morphology (%) | 39.58±7.64 | 38.5±7.18 | 36.9±8.37 |
Table 2.
Correlations between semen parameters and seminal plasma CoQ10 and antioxidant levels post-CoQ10 treatment
References
1. Hofny ER, Ali ME, Abdel-Hafez HZ, Kamal Eel-D, Mohamed EE, Abd El-Azeem HG, et al. Semen parameters and hormonal profile in obese fertile and infertile males. Fertil Steril 2010;94:581-4.


2. Gurunath S, Pandian Z, Anderson RA, Bhattacharya S. Defining infertility: a systematic review of prevalence studies. Hum Reprod Update 2011;17:575-88.


4. Brugh VM 3rd, Lipshultz LI. Male factor infertility: evaluation and management. Med Clin North Am 2004;88:367-85.

5. Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, Behre HM, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update 2010;16:231-45.


6. Adamopoulos DA. Medical treatment of idiopathic oligozoospermia and male factor subfertility. Asian J Androl 2000;2:25-32.

7. Cavallini G, Ferraretti AP, Gianaroli L, Biagiotti G, Vitali G. Cinnoxicam and L-carnitine/acetyl-L-carnitine treatment for idiopathic and varicocele-associated oligoasthenospermia. J Androl 2004;25:761-72.

8. Bisht S, Faiq M, Tolahunase M, Dada R. Oxidative stress and male infertility. Nat Rev Urol 2017;14:470-85.



9. Alahmar AT, Sengupta P. Impact of coenzyme Q10 and selenium on seminal fluid parameters and antioxidant status in men with idiopathic infertility. Biol Trace Elem Res 2021;199:1246-52.



10. Ahmadi S, Bashiri R, Ghadiri-Anari A, Nadjarzadeh A. Antioxidant supplements and semen parameters: an evidence based review. Int J Reprod Biomed 2016;14:729-36.




11. Agarwal A, Mulgund A, Alshahrani S, Assidi M, Abuzenadah AM, Sharma R, et al. Reactive oxygen species and sperm DNA damage in infertile men presenting with low level leukocytospermia. Reprod Biol Endocrinol 2014;12:126.



12. Alahmar AT, Sengupta P, Dutta S, Calogero AE. Coenzyme Q10, oxidative stress markers, and sperm DNA damage in men with idiopathic oligoasthenoteratospermia. Clin Exp Reprod Med 2021;48:150-5.




13. Agarwal A, Majzoub A, Baskaran S, Panner Selvam MK, Cho CL, Henkel R, et al. Sperm DNA fragmentation: a new guideline for clinicians. World J Mens Health 2020;38:412-71.




14. Agarwal A, Nallella KP, Allamaneni SS, Said TM. Role of antioxidants in treatment of male infertility: an overview of the literature. Reprod Biomed Online 2004;8:616-27.


15. Lipovac M, Bodner F, Imhof M, Chedraui P. Comparison of the effect of a combination of eight micronutrients versus a standard mono preparation on sperm parameters. Reprod Biol Endocrinol 2016;14:84.




16. Safarinejad MR, Safarinejad S. Efficacy of selenium and/or N-acetyl-cysteine for improving semen parameters in infertile men: a double-blind, placebo controlled, randomized study. J Urol 2009;181:741-51.


17. Panner Selvam MK, Agarwal A, Henkel R, Finelli R, Robert KA, Iovine C, et al. The effect of oxidative and reductive stress on semen parameters and functions of physiologically normal human spermatozoa. Free Radic Biol Med 2020;152:375-85.


18. Hofman-Bang C, Rehnqvist N, Swedberg K, Wiklund I, Astrom H. Coenzyme Q10 as an adjunctive in the treatment of chronic congestive heart failure. J Card Fail 1995;1:101-7.

19. Alleva R, Scararmucci A, Mantero F, Bompadre S, Leoni L, Littarru GP. The protective role of ubiquinol-10 against formation of lipid hydroperoxides in human seminal fluid. Mol Aspects Med 1997;18 Suppl:S221-8.


20. Balercia G, Buldreghini E, Vignini A, Tiano L, Paggi F, Amoroso S, et al. Coenzyme Q10 treatment in infertile men with idiopathic asthenozoospermia: a placebo-controlled, double-blind randomized trial. Fertil Steril 2009;91:1785-92.


21. Alahmar AT. The impact of two doses of coenzyme Q10 on semen parameters and antioxidant status in men with idiopathic oligoasthenoteratozoospermia. Clin Exp Reprod Med 2019;46:112-8.




22. Vishvkarma R, Alahmar AT, Gupta G, Rajender S. Coenzyme Q10 effect on semen parameters: profound or meagre? Andrologia 2020;52:e13570.



23. Imamovic Kumalic S, Pinter B. Review of clinical trials on effects of oral antioxidants on basic semen and other parameters in idiopathic oligoasthenoteratozoospermia. Biomed Res Int 2014;2014:426951.


24. Safarinejad MR, Safarinejad S, Shafiei N, Safarinejad S. Effects of the reduced form of coenzyme Q10 (ubiquinol) on semen parameters in men with idiopathic infertility: a double-blind, placebo controlled, randomized study. J Urol 2012;188:526-31.


25. Harden M, Friede T. Sample size calculation in multi-centre clinical trials. BMC Med Res Methodol 2018;18:156.




26. World Health Organization. WHO laboratory manual for the examination and processing of human semen. 5th ed. Geneva: World Health Organization; 2010.
27. World Health Organization. WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction. Cambridge: Cambridge University Press; 1999.

28. Magnani L, Gaydou EM, Hubaud JC. Spectrophotometric measurement of antioxidant properties of flavones and flavonols against superoxide anion. Anal Chim Acta 2000;411:209-16.

29. Venkatesh S, Shamsi MB, Dudeja S, Kumar R, Dada R. Reactive oxygen species measurement in neat and washed semen: comparative analysis and its significance in male infertility assessment. Arch Gynecol Obstet 2011;283:121-6.


30. Li K, Shi Y, Chen S, Li W, Shang X, Huang Y. Determination of coenzyme Q10 in human seminal plasma by high-performance liquid chromatography and its clinical application. Biomed Chromatogr 2006;20:1082-6.


31. Alahmar AT. The effects of oral antioxidants on the semen of men with idiopathic oligoasthenoteratozoospermia. Clin Exp Reprod Med 2018;45:57-66.




32. Safarinejad MR. Efficacy of coenzyme Q10 on semen parameters, sperm function and reproductive hormones in infertile men. J Urol 2009;182:237-48.


33. Safarinejad MR. The effect of coenzyme Q11 supplementation on partner pregnancy rate in infertile men with idiopathic oligoasthenoteratozoospermia: an open-label prospective study. Int Urol Nephrol 2012;44:689-700.



34. Cakiroglu B, Eyyupoglu SE, Gozukucuk R, Uyanik BS. Ubiquinol effect on sperm parameters in subfertile men who have astheno-teratozoospermia with normal sperm concentration. Nephrourol Mon 2014;6:e16870.



35. Alahmar AT, Calogero AE, Sengupta P, Dutta S. Coenzyme Q10 improves sperm parameters, oxidative stress markers and sperm DNA fragmentation in infertile patients with idiopathic oligoasthenozoospermia. World J Mens Health 2021;39:346-51.



36. Salvio G, Cutini M, Ciarloni A, Giovannini L, Perrone M, Balercia G. Coenzyme Q10 and male infertility: a systematic review. Antioxidants (Basel) 2021;10:874.



37. Lafuente R, Gonzalez-Comadran M, Sola I, Lopez G, Brassesco M, Carreras R, et al. Coenzyme Q10 and male infertility: a meta-analysis. J Assist Reprod Genet 2013;30:1147-56.




38. Pala R, Orhan C, Tuzcu M, Sahin N, Ali S, Cinar V, et al. Coenzyme Q10 supplementation modulates NFκB and Nrf2 pathways in exercise training. J Sports Sci Med 2016;15:196-203.


39. Li X, Zhan J, Hou Y, Chen S, Hou Y, Xiao Z, et al. Coenzyme Q10 suppresses oxidative stress and apoptosis via activating the Nrf-2/NQO-1 and NF-κB signaling pathway after spinal cord injury in rats. Am J Transl Res 2019;11:6544-52.


40. Alahmar AT, Singh R. Comparison of the effects of coenzyme Q10 and Centrum multivitamins on semen parameters, oxidative stress markers, and sperm DNA fragmentation in infertile men with idiopathic oligoasthenospermia. Clin Exp Reprod Med 2022;49:49-56.




41. Huang C, Cao X, Pang D, Li C, Luo Q, Zou Y, et al. Is male infertility associated with increased oxidative stress in seminal plasma?: a-meta analysis. Oncotarget 2018;9:24494-513.



42. Ko EY, Sabanegh ES. The role of nutraceuticals in male fertility. Urol Clin North Am 2014;41:181-93.


43. Humaidan P, Haahr T, Povlsen BB, Kofod L, Laursen RJ, Alsbjerg B, et al. The combined effect of lifestyle intervention and antioxidant therapy on sperm DNA fragmentation and seminal oxidative stress in IVF patients: a pilot study. Int Braz J Urol 2022;48:131-56.


44. Balercia G, Mancini A, Paggi F, Tiano L, Pontecorvi A, Boscaro M, et al. Coenzyme Q10 and male infertility. J Endocrinol Invest 2009;32:626-32.



45. Balercia G, Mosca F, Mantero F, Boscaro M, Mancini A, Ricciardo-Lamonica G, et al. Coenzyme Q(10) supplementation in infertile men with idiopathic asthenozoospermia: an open, uncontrolled pilot study. Fertil Steril 2004;81:93-8.


46. Alahmar AT, Calogero AE, Singh R, Cannarella R, Sengupta P, Dutta S. Coenzyme Q10, oxidative stress, and male infertility: a review. Clin Exp Reprod Med 2021;48:97-104.




47. Balercia G, Moretti S, Vignini A, Magagnini M, Mantero F, Boscaro M, et al. Role of nitric oxide concentrations on human sperm motility. J Androl 2004;25:245-9.


48. Nadjarzadeh A, Sadeghi MR, Amirjannati N, Vafa MR, Motevalian SA, Gohari MR, et al. Coenzyme Q10 improves seminal oxidative defense but does not affect on semen parameters in idiopathic oligoasthenoteratozoospermia: a randomized double-blind, placebo controlled trial. J Endocrinol Invest 2011;34:e224-8.








