|
 |
- Search
| Clin Exp Reprod Med > Volume 45(2); 2018 > Article |
|
Abstract
Objective
Prompt diagnosis and management are essential for saving the adnexal organs from infarction in cases of ovarian torsion (OT). This study aimed to determine the diagnostic significance of signal peptide, complement C1r/C1s, Uegf, and Bmp1 (CUB), and epidermal growth factor-like domain-containing protein-1 (SCUBE-1) levels in cases of OT, an emergent ischemic condition, and the relationship of SCUBE-1 with oxidative stress parameters.
Methods
This prospective study was conducted among 15 OT patients and 20 age- and gravidity-matched healthy women. SCUBE-1 serum concentrations were determined by using enzyme-linked immunosorbent assays. In addition, oxidative stress was evaluated by measuring the serum levels of advanced oxidation protein products (AOPP), ferric reducing ability of plasma (FRAP), and glutathione (GSH).
Results
The SCUBE-1 titers were significantly higher in the patients with OT than in the controls (p=0.008). In addition, serum FRAP and GSH levels were significantly lower in the OT patients than in the controls (p<0.001 for both). Serum AOPP levels were higher in the OT patients, but this trend was not statistically significant (p>0.05). Furthermore, there were no correlations between SCUBE-1 levels and age, gravidity, parity, cyst size, and AOPP, FRAP, or GSH levels (p>0.05).
Ovarian torsion (OT) is a rare and emergent condition defined as the rotation of the ovarian peduncle around its own axis, completely or incompletely. Although OT can occur at any stage of life, it is most common during the reproductive years. Therefore, the prompt management of OT is very important for the reproductive health of women affected by this condition. Unfortunately, the diagnosis and surgical intervention in OT cases may be delayed because OT can present with nonspecific clinical findings, such as the sudden onset of severe pain, vomiting, nausea, leukocytosis, fever, and a palpable adnexal mass [1]. Overall, the pathological and radiological findings vary based on the extent of the vascular structures involved. Reduced venous return, ovarian enlargement, edema, and interstitial hemorrhage appear during the very beginning of torsion. Subsequently, the arterial flow of the ovary is disrupted, and ovarian ischemia and necrosis occur [2]. Thus, the diagnosis may be delayed.
Although several imaging techniques are available, such as grayscale ultrasonography (USG), Doppler USG, and computed tomography (CT), the correct diagnosis of OT is difficult because of its nonspecific findings [3,4]. Ovarian cysts should be considered first in the differential diagnosis because patients with ruptured ovarian cysts present with acute abdominal signs resembling OT that are difficult to differentiate with certainty preoperatively [5]. Therefore, several markers, such as ischemia-modified albumin and interleukin (IL)-6 levels, have been studied to determine whether they can be used to make an early diagnosis of OT [6,7].
Signal peptide, complement C1r/C1s, Uegf, and Bmp1 (CUB), and epidermal growth factor-like domain-containing protein-1 (SCUBE-1) is a newly described cell surface protein. The SCUBE-1 expressed in endothelial cells and activated platelets has been found to be responsible for ischemic complications [8,9]. Previously, SCUBE-1 levels and oxidative stress parameters have been investigated in various ischemic diseases, such as acute coronary syndrome, acute ischemic stroke, and acute mesenteric ischemia [9,10]. However, the role of SCUBE-1 and its relationship with oxidative stress parameters has not previously been studied in patients with OT. To the best of our knowledge, this is the first study to evaluate the relationship of serum SCUBE-1 levels with oxidative stress parameters, with the goal of assessing SCUBE-1 as a biomarker for diagnosing OT.
This prospective study was conducted between June 2015 and July 2017 at the Departments of Obstetrics and Gynecology and Clinical Biochemistry of the Harran University Medical Faculty in Sanliurfa, Turkey. This study conformed with the principles of the 2008 Declaration of Helsinki and was approved by the local ethics committee of the Harran University Medical Faculty (15.06.2015/No. 121). Detailed information was provided to all the women enrolled in this study, and written informed consent was provided by all participants.
This study included 15 women who underwent laparotomies or laparoscopies due to OT and 20 healthy age- and gravidity-matched controls with no history of abdominal pain. Individuals with diabetes or hypertension, pregnant women, and patients with a tubo-ovarian abscess, ruptured ovarian cyst, endometriosis, or hydrosalpinx were excluded. The diagnosis of suspicion of OT was made based on USG results (Voluson 730 Expert scanner; GE Healthcare, Milwaukee, WI, USA) showing ovarian enlargement and the absence of Doppler velocity in the ovarian peduncle. In addition, patients' defense was evaluated during the USG examination. The preoperative diagnosis of OT was confirmed intraoperatively in all patients with adnexal torsion.
Each woman's age, marital status, gravidity, parity, blood pressure, and operation type were documented. Venous blood samples were collected preoperatively when the OT diagnosis was made for biochemical comparisons between the groups.
The serum was obtained via centrifugation at 4,000 g for 15 minutes at 4Ōäā. The plasma samples were immediately stored at ŌłÆ80Ōäā until analysis.
A human SCUBE-1 enzyme-linked immunosorbent assay kit (Cat no. E-EL-H5405; Elabscience Biotechnology, Houston, TX, USA) was used, and the results were measured in nanograms per milliliter. The other blood analyses were performed within 2 hours of the blood sampling using a hematology analyzer (CELL-DYN Ruby; Abbot, Abbott Park, IL, USA) in the biochemistry laboratory of Harran University Medical Faculty.
Glutathione (GSH) levels were assessed through a reaction with ophthalaldehyde (1 mg/mL ophthalaldehyde in methanol) using a modified version of the technique of Koyuncu et al. [11]. The GSH samples were assessed via a microplate reader (SpectraMax M5; Molecular Devices, San Jose, CA, USA) with 345 nm excitation and 425 nm emission. The results were expressed as nanomoles per milliliter in the serum.
The ferric reducing ability of plasma (FRAP) assay has been presented as a novel method for assessing antioxidant power, in which the ferric to ferrous ion reduction at a low pH causes a colored ferrous-tripyridyltriazine complex to form [12]. The results were expressed as micromoles per liter in the serum.
The spectrophotometric determination of the advanced oxidation protein products (AOPP) was performed using a modification of the method of Witko et al. [13]. AOPP concentrations were expressed as micromoles per liter of chloramine-T equivalents. Until the study ended, the researchers were unaware of the serum levels of the studied biomarkers in relation to the participants' clinical characteristics (age, gravidity, etc.).
All analyses were performed using IBM SPSS ver. 20.0 (IBM Corp., Armonk, NY, USA). All data were expressed as mean and standard deviation. Comparisons of the groups were performed with the nonparametric Mann-Whitney U-test. Correlations between the parameters were assessed using the Spearman correlation test. Differences in the groups' biochemical parameters were analyzed using the independent-samples t-test. A p-value<0.05 was considered to indicate statistical significance.
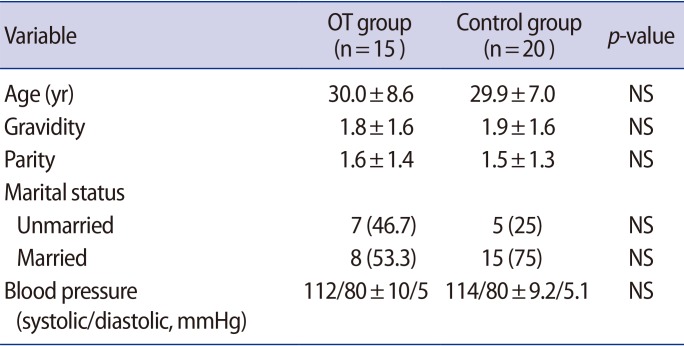
The demographic and clinical features of the groups are presented in Table 1. The mean cyst size was 66.60┬▒23.76 mm, and the biggest cyst was 125 mm in the OT group. Most of the cysts were located in the left adnexal region (11 patients). A laparoscopy was performed in eight patients (53.3%), and a laparotomy was performed in seven (46.7%). In terms of the treatment modality, detorsion with cyst excision was performed in nine patients (60%) and salpingo-oophorectomy was performed in six (40%). The mean torsion time was 24.4┬▒14.8 hours (range, 7ŌĆō48 hours). We detected defense and rebound positivity in all patients during the USG examinations. In addition, no participants in either group had a previous history of pelvic operation. All participants had normal arterial blood pressure readings.
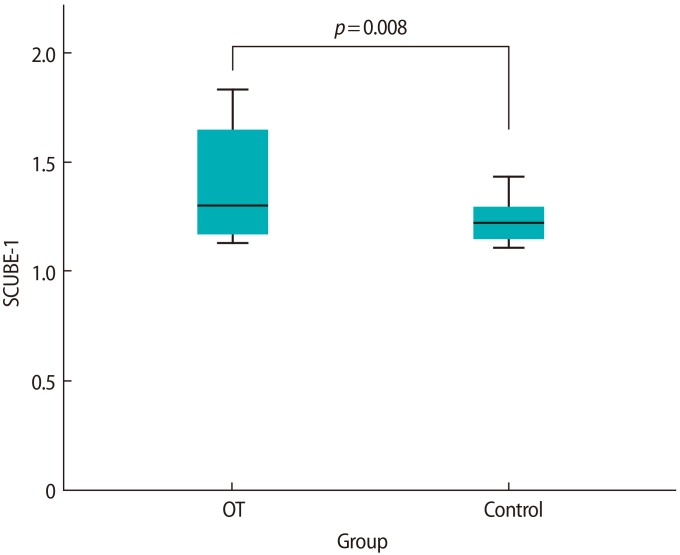
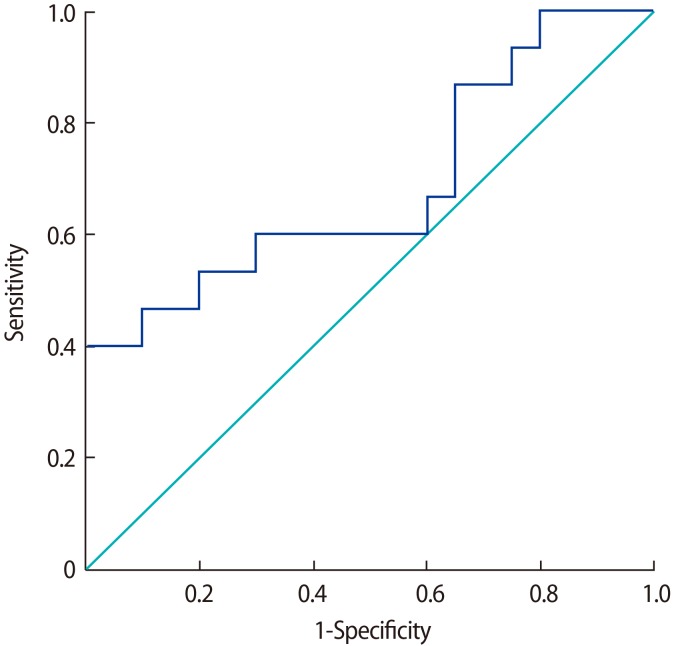
The preoperative circulating SCUBE-1 levels were significantly higher in the OT group than in the control group (1.40┬▒0.25 ng/mL vs. 1.22┬▒0.09 ng/mL; p=0.008) (Figure 1). A receiver operating characteristic (ROC) analysis was performed and shown in Figure 2. The area under the curve (AUC) was 0.68 (95% confidence interval [CI], 0.50ŌĆō0.87). According to the ROC analysis, the cutoff value of 1.13 ng/mL for SCUBE-1 had a sensitivity of 80% and specificity of 93.3%. When we used a cutoff value of 1.20 ng/mL for SCUBE-1, the values of both sensitivity and specificity were 60%.
The serum AOPP level was slightly higher in the OT patients than in the control group (45.4┬▒20.5 ┬Ąmol/L vs. 37.2┬▒8.0 ┬Ąmol/L, p>0.05); however, this difference was not statistically significant. The serum FRAP and GSH concentrations were significantly lower in the OT patients than in the controls (263.3┬▒43.3 ┬Ąmol/L vs. 323.0┬▒44.4 ┬Ąmol/L and 6.0┬▒0.8 nmol/mL vs. 7.9┬▒1.5 nmol/mL, respectively; p<0.001 for both). Spearman correlation analysis showed that there were no correlations between SCUBE-1 levels and AOPP, FRAP, GSH levels, and age, gravidity, parity, torsion time, or ovarian cyst size (all p>0.05).
In this study, we found significantly higher serum SCUBE-1 levels and significantly lower levels of antioxidant parameters (FRAP and GSH) in patients with OT. AOPP levels were also higher in the OT patients, but this trend did not show statistical significance.
No previous study has evaluated SCUBE-1 levels in patients with adnexal torsion. Nevertheless, in many studies, elevated SCUBE-1 levels have been found in acute ischemic events, such as acute coronary syndrome, ischemic stroke, pulmonary embolism, and acute mesenteric ischemia [9,10,14,15]. Ulusoy et al. [16] reported elevated SCUBE-1 levels in hemodialysis patients with no clinically acute ischemic events. They suggested that various factors, such as sex, systolic and diastolic blood pressure, blood urea nitrogen, and creatinine, could explain this elevation. In addition, elevated SCUBE-1 levels have been reported in some chronic diseases, including hypertension and Hashimoto thyroiditis [17,18]. Therefore, we excluded patients with chronic diseases (as mentioned above), and the blood pressure readings of our patients were within the normal range.
USG is the first-line imaging modality used in the diagnosis of OT; however, its sensitivity and specificity have been reported to be 46% and 74%, respectively [3]. The appearance of arterial or venous flow in the Doppler USG can not rule out the diagnosis of OT because of the dual blood supply of the ovaries (from both the ovarian artery and the arterial branches of the uterus) [4]. Kato et al. [19] and Fujii et al. [20] showed that a diffusion-weighted imaging modality had high sensitivity for the diagnosis of the early stages of OT. Although some multimodal imaging techniques can be used to diagnose OT, an accurate diagnosis is difficult due to the nonspecific findings and the long list of differential diagnoses. Ruptured ovarian cysts should be considered first in the differential diagnosis because patients with ruptured ovarian cysts present with acute abdominal signs, with some resemblance to OT, and are difficult to clearly differentiate preoperatively [5]. All participants in this study underwent conventional USG and Doppler USG examinations, while CT was only performed for a patient with a huge abdominopelvic mass in order to exclude other differential diagnoses, such as a ruptured ovarian cyst. Because our control group was composed of healthy women without any abdominal pain, SCUBE-1 elevation may not be helpful to differentiate OT from other causes of acute abdominal pain. Therefore, we believe that more comprehensive studies, including suitable control groups, will be necessary to elucidate the role of SCUBE-1 in such clinical situations.
The laparoscopic approach is currently preferred for many OT patients [21]. According to Galinier et al. [22], a conservative approach toward the ovaries after detorsion is safe and effective. We chose laparoscopic interventions, as an early and less-invasive method of performing detorsion, for eight women in order to maintain the integrity of their ovaries. The correction of the ovarian circulation after detorsion disrupts the tissue injury and leads to a pathological process known as ischemia/reperfusion (I/R) injury, which is caused by oxidative stress. Oxidative stress creates an imbalance between the production of reactive oxygen species and the detoxification of the body's reactive intermediates. This imbalance leads to the overproduction of free radicals, which can be seen in many ischemic diseases, or to a decrease in the antioxidant enzyme capacity [23]. Many studies have shown that oxidative stress is activated by the OT process, and have recommended using antioxidant agents (e.g., selenium or N-acetylcysteine) to minimize the I/R injury to the ovaries during or before reperfusion [24,25]. The finding of our study that levels of antioxidant agents (FRAP and GSH) were significantly lower in OT patients suggests that these antioxidant molecules were consumed by the body in cases of OT.
However, we found no correlations between SCUBE-1 levels and AOPP, FRAP, or GSH levels. The lack of a correlation between SCUBE-1 levels and the oxidant and antioxidant parameters might have been due to the small sample size of this study. No previous study in the literature has published findings regarding the relationship between SCUBE-1 and oxidative stress.
In a recent experimental study, Ozler et al. [26] showed a significant decrease in ovarian reserve after conservative adnexal torsion surgery by measuring the serum levels of anti-M├╝llerian hormone, inhibin B, and estradiol in rats. They argued that conservative surgery alone was insufficient to preserve ovarian function. Because a prolonged duration of torsion may lead to a loss in ovarian function, an early diagnosis is important to protect the ovary from ischemia and necrosis [5]. Daponte et al. [6] demonstrated that increases in certain inflammatory markers in the serum, such as IL-6, can play an important role in the early diagnosis of patients with adnexal torsion. In a clinical study, Dai et al. [9] reported that SCUBE-1 was detectable in the serum as early as within 6 hours after the onset of ischemic symptoms, suggesting that it could be a good marker for acute ischemic diseases. Although we did not establish a correlation between the degree of torsion and the measured SCUBE-1 levels, we showed that serum SCUBE-1 levels were elevated within 48 hours after the clinical onset of the disease. Hence, we suggest that SCUBE-1 might be a valuable marker in acute OT cases. However, the AUC value of 0.68 (95% CI, 0.50ŌĆō0.87) detected in the ROC analysis indicated that SCUBE-1 had a poor discriminatory power as a diagnostic test for OT. This weakness may have been due to the small number of OT patients or variations in the duration and severity of the disease in our study.
Several studies have shown that some serum biomarkers, such as serum d-dimer, IL-6, IL-8, tumor necrosis factor-alpha (TNF-╬▒), heat shock protein 70, and ischemia-modified albumin, can play a role in the early detection of OT [7,27,28,29]. Kart et al. [29] studied the diagnostic value of plasma d-dimer levels in an experimental rat OT model and found a significant correlation between plasma d-dimer levels and the histopathological damage scores of ovarian injuries. Cilgin et al. [28] investigated the role of serum heat shock protein 70 in a rat model and suggested that levels of this protein seemed to be a useful serum marker for the early detection of OT. Aran et al. [7] showed that serum ischemia-modified albumin levels were correlated with the histological scores of the specimens in an experimental rat OT model. In our previous experimental rat study, we detected an increase in several oxidative stress parameters (total oxidant status, paraoxonase, and lipid hydroperoxide) and TNF-╬▒ levels. We also found that these markers were correlated with the histopathological damage scores of ovarian injuries [25].
We chose to study SCUBE-1, a new ischemic marker, during the early stages of OT and found that it may be a useful marker for making an early diagnosis. However, this study had some limitations. First, the number of subjects included was relatively low because an OT is a very rare condition. Second, we did not present data on the levels of other ischemic markers, such as ischemia-modified albumin and malondialdehyde. Third, no data were obtained regarding time-dependent changes in SCUBE-1 levels and oxidative stress parameters.
Although many studies on OT have been case reports or experimental studies, we used a prospective study design, and the characteristics of the patients (age, marital status, and relative torsion time) were homogeneous in the study groups. In addition, this is the first study to evaluate SCUBE-1 levels in OT patients. These represent some of the strengths of our study.
In conclusion, we showed that elevated serum SCUBE-1 levels may be an acute ischemic marker in patients with OT. In addition, SCUBE-1 levels can be used effectively to support the early diagnosis of OT. However, we believe that further clinical research is required to validate our findings, to improve their implementation in practice, and to shorten the delay in the diagnosis of OT.
Acknowledgments
We would like to thank the Department of Clinical Biochemistry of Harran University Medical Faculty.
References
1. Bekci T, Polat AV, Aslan K, Tomak L, Ceyhan Bilgici M, Danaci M. Diagnostic performance of diffusion-weighted MRI in the diagnosis of ovarian torsion: comparison of torsed and nonaffected ovaries. Clin Imaging 2016;40:1029-1033.PMID: 27348059.


2. Albayram F, Hamper UM. Ovarian and adnexal torsion: spectrum of sonographic findings with pathologic correlation. J Ultrasound Med 2001;20:1083-1089.PMID: 11587015.


3. Wilkinson C, Sanderson A. Adnexal torsion: a multimodality imaging review. Clin Radiol 2012;67:476-483.PMID: 22137723.


4. Chang HC, Bhatt S, Dogra VS. Pearls and pitfalls in diagnosis of ovarian torsion. Radiographics 2008;28:1355-1368.PMID: 18794312.


5. Shiota M, Kotani Y, Umemoto M, Tobiume T, Hoshiai H. Preoperative differentiation between tumor-related ovarian torsion and rupture of ovarian cyst preoperatively diagnosed as benign: a retrospective study. J Obstet Gynaecol Res 2013;39:326-329.PMID: 22690912.


6. Daponte A, Pournaras S, Hadjichristodoulou C, Lialios G, Kallitsaris A, Maniatis AN, et al. Novel serum inflammatory markers in patients with adnexal mass who had surgery for ovarian torsion. Fertil Steril 2006;85:1469-1472.PMID: 16616744.


7. Aran T, Guven S, Unsal MA, Alver A, Mentese A, Yulug E. Serum ischemia-modified albumin as a novel marker of ovarian torsion: an experimental study. Eur J Obstet Gynecol Reprod Biol 2010;150:72-75.PMID: 20172646.


8. Yang RB, Ng CK, Wasserman SM, Colman SD, Shenoy S, Mehraban F, et al. Identification of a novel family of cell-surface proteins expressed in human vascular endothelium. J Biol Chem 2002;277:46364-46373.PMID: 12270931.


9. Dai DF, Thajeb P, Tu CF, Chiang FT, Chen CH, Yang RB, et al. Plasma concentration of SCUBE1, a novel platelet protein, is elevated in patients with acute coronary syndrome and ischemic stroke. J Am Coll Cardiol 2008;51:2173-2180.PMID: 18510966.


10. Turkmen S, Mentese S, Mentese A, Sumer AU, Saglam K, Yulug E, et al. The value of signal peptide-CUB-EGF domain-containing protein 1 and oxidative stress parameters in the diagnosis of acute mesenteric ischemia. Acad Emerg Med 2013;20:257-264.PMID: 23517257.


11. Koyuncu I, Kocyigit A, Gonel A, Arslan E, Durgun M. The protective effect of naringenin-oxime on cisplatin-induced toxicity in rats. Biochem Res Int 2017;2017:9478958. PMID: 28932603.




12. Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of ŌĆ£antioxidant powerŌĆØ: the FRAP assay. Anal Biochem 1996;239:70-76.PMID: 8660627.


13. Witko V, Nguyen AT, Descamps-Latscha B. Microtiter plate assay for phagocyte-derived taurine-chloramines. J Clin Lab Anal 1992;6:47-53.PMID: 1542083.


14. Sonmez E, Turkdogan KA, Karabacak M, Civelek C, Yilmaz C, Ozer OF, et al. The diagnostic role of signal peptide-C1r/C1s, Uegf, and Bmp1-epidermal growth factor domain-containing protein 1 in non-ST-elevation acute coronary syndrome. Am J Emerg Med 2015;33:21-24.PMID: 25445868.


15. Turkmen S, Sahin A, Gunaydin M, Sahin S, Mentese A, Turedi S, et al. The value of signal peptide-CUB-EGF domain-containing protein-1 (SCUBE1) in the diagnosis of pulmonary embolism: a preliminary study. Acad Emerg Med 2015;22:922-926.PMID: 26202675.


16. Ulusoy S, Ozkan G, Mentese A, Yavuz A, Karahan SC, Sumer AU. Signal peptide-CUB-EGF domain-containing protein 1 (SCUBE1) level in hemodialysis patients and parameters affecting that level. Clin Biochem 2012;45:1444-1449.PMID: 22874483.


17. Ozkan G, Ulusoy S, Mentese A, Karahan SC, Cansiz M. New marker of platelet activation, SCUBE1, is elevated in hypertensive patients. Am J Hypertens 2013;26:748-753.PMID: 23443724.



18. Bilir B, Soysal-Atile N, Ekiz Bilir B, Yilmaz I, Bali I, Altintas N, et al. Evaluation of SCUBE-1 and sCD40L biomarkers in patients with hypothyroidism due to Hashimoto's thyroiditis: a single-blind, controlled clinical study. Eur Rev Med Pharmacol Sci 2016;20:407-413.PMID: 26914113.

19. Kato H, Kanematsu M, Uchiyama M, Yano R, Furui T, Morishige K. Diffusion-weighted imaging of ovarian torsion: usefulness of apparent diffusion coefficient (ADC) values for the detection of hemorrhagic infarction. Magn Reson Med Sci 2014;13:39-44.PMID: 24492742.


20. Fujii S, Kaneda S, Kakite S, Kanasaki Y, Matsusue E, Harada T, et al. Diffusion-weighted imaging findings of adnexal torsion: initial results. Eur J Radiol 2011;77:330-334.PMID: 19716670.


21. Zweizig S, Perron J, Grubb D, Mishell DR Jr. Conservative management of adnexal torsion. Am J Obstet Gynecol 1993;168(6 Pt 1): 1791-1795.PMID: 8317522.


22. Galinier P, Carfagna L, Delsol M, Ballouhey Q, Lemasson F, Le Mandat A, et al. Ovarian torsion. Management and ovarian prognosis: a report of 45 cases. J Pediatr Surg 2009;44:1759-1765.PMID: 19735822.


23. Iadecola C, Alexander M. Cerebral ischemia and inflammation. Curr Opin Neurol 2001;14:89-94.PMID: 11176223.


24. Bozkurt S, Arikan DC, Kurutas EB, Sayar H, Okumus M, Coskun A, et al. Selenium has a protective effect on ischemia/reperfusion injury in a rat ovary model: biochemical and histopathologic evaluation. J Pediatr Surg 2012;47:1735-1741.PMID: 22974615.


25. Kilic A, Uyanikoglu H, Incebiyik A. Protective effect of N-acetylcystein and resveratrol on ischemia-reperfusion injury in rat ovary. Dicle Med J 2016;43:229-236.
26. Ozler A, Turgut A, Soydinc HE, Sak ME, Evsen MS, Alabalik U, et al. The biochemical and histologic effects of adnexal torsion and early surgical intervention to unwind detorsion on ovarian reserve: an experimental study. Reprod Sci 2013;20:1349-1355.PMID: 23585344.


27. Christopoulos G, Goubet S, Kelly T. Interleukin-6 for the diagnosis of ovarian torsion: a systematic review and meta-analysis. J Obstet Gynaecol 2013;33:438-441.PMID: 23815191.


28. Cilgin H, Simsek M, Bal R. Can adnexal torsion be predicted by measuring plasma heat shock protein 70 level? An experimental study. Arch Gynecol Obstet 2017;296:941-946.PMID: 28866782.



29. Kart C, Aran T, Guven S, Karahan SC, Yulug E. Acute increase in plasma D-dimer level in ovarian torsion: an experimental study. Hum Reprod 2011;26:564-568.PMID: 21242148.


Figure┬Ā1
Serum SCUBE-1 levels in the ovarian torsion (OT) and control groups. SCUBE-1, signal peptide, complement C1r/C1s, Uegf, and Bmp1 (CUB), and epidermal growth factor-like domain-containing protein-1.
Figure┬Ā2
Receiver operating characteristic curve analysis for serum SCUBE-1 levels in ovarian torsion patients (respective cutoff, area under the curve, sensitivity and specificity values). The area under the curve was 0.68 (95% confidence interval, 0.50ŌĆō0.87). SCUBE-1, signal peptide, complement C1r/C1s, Uegf, and Bmp1 (CUB), and epidermal growth factor-like domain-containing protein-1.
-
METRICS

- Related articles in Clin Exp Reprod Med
-
Useful Biomarkers for the Diagnosis of Endometriosis.2010 March;37(1)